Endorphins and opioid pathways

Neuro peptides and opiate receptors
Role in addiction
The endorphin system and the role it plays in the brain is one of the more complex and newer frontiers in neuroscience. This post is a short introduction to some of the basic information I have found and how it pertains to the pathophysiology of addiction.

*note: the war to stamp out opium does not seem to be going well.
The known use of the latex obtained from the seed pod of Papaver Somniferum, the opium poppy, for both medicinal and non medicinal purposes is as old as recorded history. More recently the refined extracts, morphine, codeine, semisynthetic heroin, hydrocodone and other opiate derived compounds are used both legally and illegally throughout the world. Raw opium is about 12% morphine. This can then be further refined to produce heroin.
There are both legal and illegal markets for opium, however reliable production statistics are difficult to find.
Methadone and fentanyl are both purely synthetic.
It was not until the 1970s that the neural receptor for morphine was found and shortly after neuropeptides, the endogenously produced opioids were isolated. While many questions remain unanswered the addictive properties of opioids, the function of the endogenous system, and it’s role in alcohol use disorder are beginning to come into focus.


Radiolabeled agents have been developed with specific binding affinity for different opioid receptors. PET images above reflect distribution and concentration of receptor types.
- Mu
- Kappa
- Delta
- Nocioceptin – Orphanin
Note the predominance of Mu receptors in central brain structures such as the Caudate, Amygdala, midbrain, and Striatum, areas important in the reward pathway and other functions. Opiate drugs have strong affinity for Mu receptors.

While opioid receptors are widely distributed in the brain they are produced only in a few select nerve bundles, the hypothalamus and the anterior pituitary. Endorphins are produced in the pituitary in response to Corticotropin releasing factor from the hypothalamus.

This shows the chemical structure of beta-endorphin. Peptides are protein like strings of amino acids with complex three dimensional structures. In that respect and others they differ from small neurotransmitters like dopamine or serotonin.
Shown is a diagram of beta-endorphin and a morphine molecule binding to Mu-receptors. Another difference from neurotransmitters is that these peptides do not have reuptake transporters. They degrade quickly in the body which is a challenge in research.


Peptides like beta-endorphin are cleaved from segments of larger precursors present in the anterior pituitary.

I feel happier already.
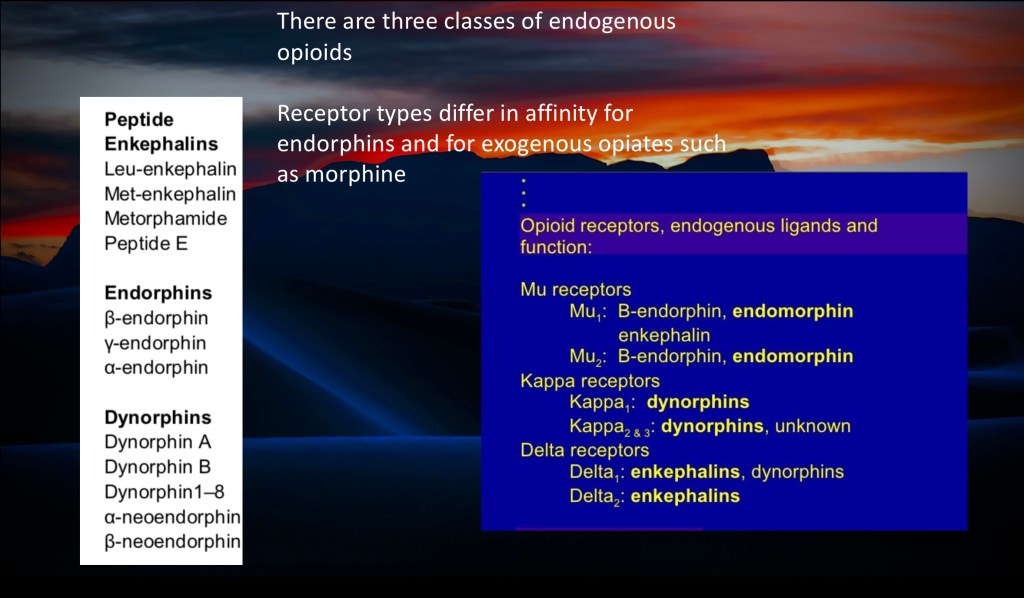
There are subtypes of peptides and subtypes of receptors.
The 3 major peptides are:
Enkephalin
Endorphin
Dynorphin
The 3 major receptors are:
Mu
Kappa
Delta
Each peptide preferentially binds to a specific receptor type. There are significant functional overlaps and differences in effect of agents, receptors, and locations.
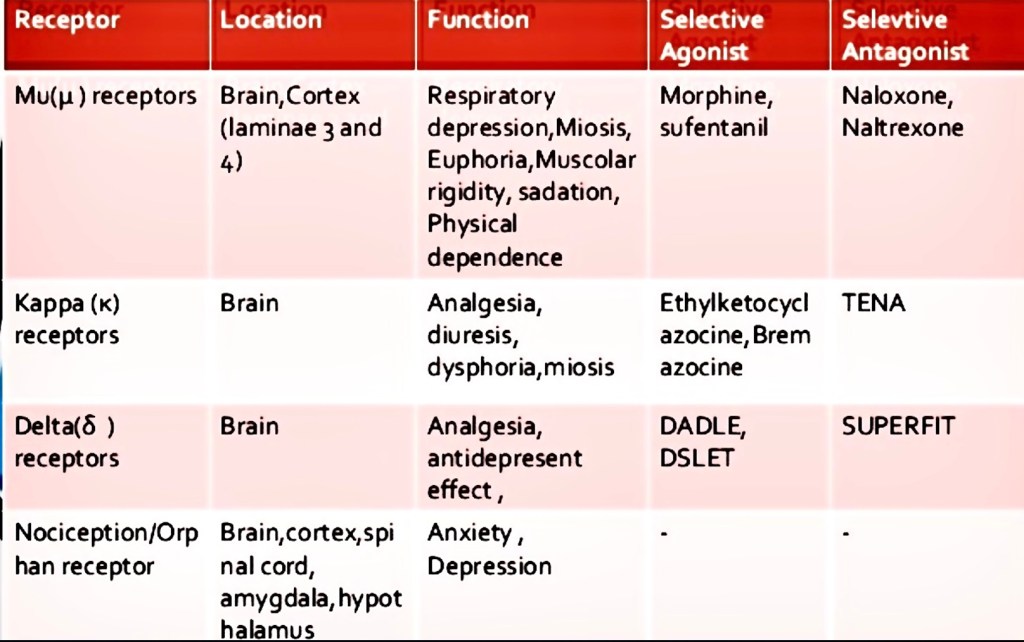
All three major receptors are involved with analgesia.
The key takeaway points are:
Opiate drugs like morphine primarily bind to Mu receptors as does b-endorphin. They are very effective pain relievers. They also sedate which can be useful. They produce euphoria and respiratory depression. Euphoria can lead to addiction. Respiratory depression can lead to death from overdose.
Naloxone is a strong Mu receptor antagonist. It reverses opioid effects completely.
Naltrexone is a weak Mu receptor antagonist. It is used in addiction treatment.
Kappa receptors bind dynorphins and produce both analgesia and dysphoria. So that isn’t good either.

Written in 2006. The holy grail has yet to be discovered.

How do opioids work?
In general opioids inhibit nerve conduction. Inhibition of pain signaling accounts for analgesic effects.
This occurs in 3 steps.
Opioid binds to receptor.
- This results in a protein mediated decrease of cAMP (cyclic adenosine mono phosphate) production. cAMP is an essential second messenger for cellular processes including production and release of neurotransmitters.
- Calcium channels close. Ca++ is needed for transmitter release.
- Potassium ion channels release K+ resulting in increased negative charge across the cell membrane. It makes it harder for the cell to initiate an action potential. The cell is now hyperpolarized.

The opiate system is an active area of investigation and is thought to play a role in alcohol use disorder in addition to opiate addiction.
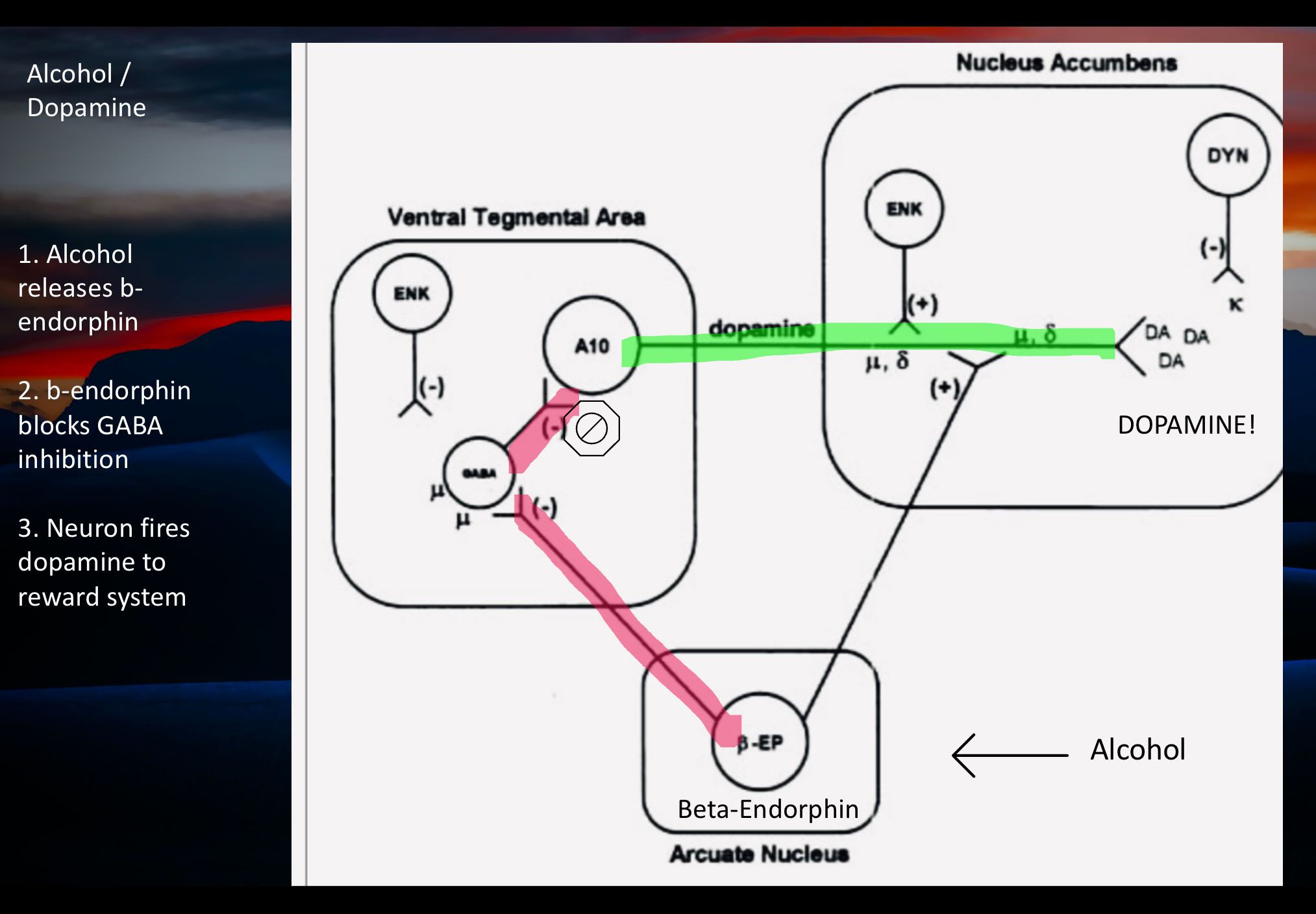
The hypothesis is that endorphins are released from the arcuate nucleus in the hypothalamus in response to alcohol. This has been demonstrated in animal models.
Endorphin then blocks GABA inhibitory neurons controlling the Dopamine reward system. This frees up positive firing inputs coming in from glutamine pathways.
The result is release of dopamine and reward activation. This mechanism is incompletely understood currently. It does explain how the partial opiate antagonist Naltrexone helps to prevent relapse in Alcohol Use Disorder.
Opioid tolerance requiring larger doses to achieve the same effect is incompletely understood at this time.

Kappa receptor dynorphin induced dysphoria is thought to further drive the addictive cycle.
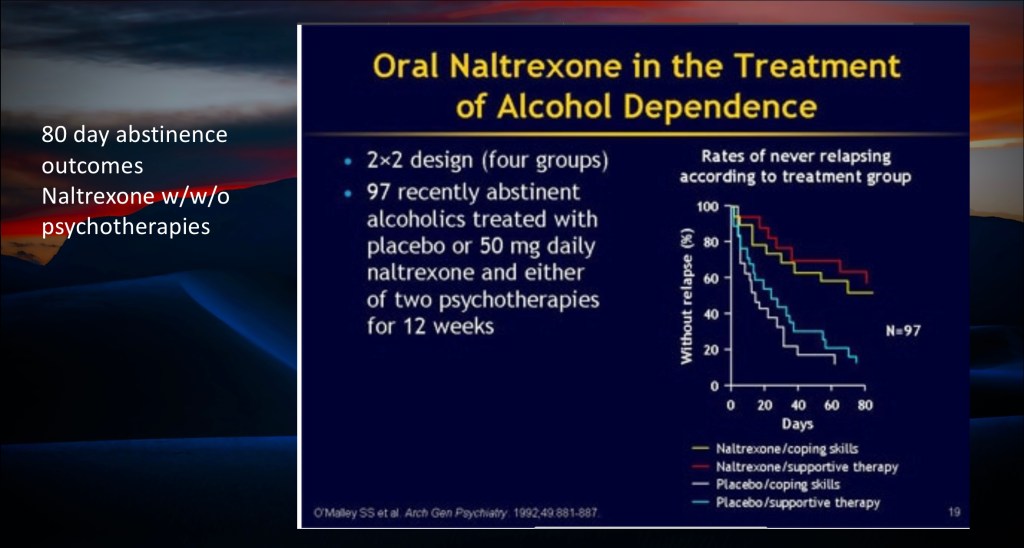
When combined with coping skills or supportive therapy Naltrexone is thought to help restore functional dopamine regulation and aid in preventing relapse.
Achieving optimal outcomes for people wanting to overcome substance use is an ongoing effort. There have been some gains with many challenges remaining.

Combined medication and psychosocial treatment and support has been shown to be most effective in several studies, however there is considerable difference between individuals. Guiding treatment providers in treatment options is an ongoing effort and a good subject for additional review.

Breathing has now been scientifically proven to beneficial.
Thank you for your consideration in reviewing this post. Any feedback is welcome.
For educational and informational purposes only. No commercial or institutional interests. This post should not be considered medical or professional advice. Images and data obtained from those freely available on the World Wide Web.
7/2023
REFERENCES
……………………
Psychopharmacology (2011) 218:229–239 DOI 10.1007/s00213-011-2337-x
Effects of acute ethanol on corticotropin-releasing hormone and β-endorphin systems at the level of the rat central amygdala
Minh P. Lam & Christina Gianoulakis
Received: 27 October 2010 / Accepted: 19 April 2011 / Published online: 20 May 2011 # Springer-Verlag 2011
………………………………………….
Understanding Endorphins and Their Importance in Pain Management
Adam S. Sprouse-Blum BA; Greg Smith BS; Daniel Sugai BA; and F. Don Parsa MD, FACS
…………….”……..
Opiate-Induced Dopamine Release Is Modulated by Severity of Alcohol Dependence: An [18F]Fallypride
Positron Emission Tomography Study
Katja N. Spreckelmeyer, Michael Paulzen, Mardjan Raptis, Thomas Baltus, Sabrina Schaffrath,
……………………………………
Stimulation of Endorphin Neurotransmission in the Nucleus Accumbens by Ethanol, Cocaine, and Amphetamine
M. Foster Olive,1 Heather N. Koenig,1 Michelle A. Nannini,1 and Clyde W. Hodge2
1Department of Neurology and Ernest Gallo Clinic and Research Center, University of California at San Francisco, Emeryville, California
…………………………………………
BRITISH JOURNAL OF PSYCHIATRY (2003), 182, 97^100
EDITORIAL
Neurobiology of addiction and implications for treatment
ANNE LINGFORD-HUGHES and DAVID NUTT
…………………….
British Journal of Pharmacology (2018) 175 2737–2749 2737
Opioid system and human emotions
Received 1 December 2016; Revised 7 March 2017; Accepted 24 March 2017
Lauri Nummenmaa1 and Lauri Tuominen2
1Turku PET Centre and Department of Psychology, University of Turku, Turku, Finland, and 2Department of Psychiatry, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA
…………………………………….
Imaging of opioid receptors in the central nervous system
Gjermund Henriksen1,2 and Frode Willoch3,4
1Nuklearmedizinische Klinik und Poliklinik, Klinikum rechts der Isar, Technische Universita« t Mu« nchen, Ismaningerstrasse 22, D- 81675 Mu« nchen, Germany,
……………………………………..
British Journal of Pharmacology (2018) 175 2737–2749 2737
Opioid system and human emotions
Received 1 December 2016; Revised 7 March 2017; Accepted 24 March 2017
Lauri Nummenmaa1 and Lauri Tuominen2
1Turku PET Centre and Department of Psychology, University of Turku, Turku, Finland, and 2Department of Psychiatry, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA
…………………………..
Positron Emission Tomography: Updates on Imaging of Addiction
Kiran Kumar Solingapuram Sai, Ph.D., Robin A. Hurley, M.D., Meghana Dodda, Katherine H. Taber, Ph.D.
Published Online:15 Oct 2019https://doi.org/10.1176/appi.neuropsych.19080169
https://neuro.psychiatryonline.org/doi/10.1176/appi.neuropsych.19080169
…………………………
Basic opioid pharmacology: an update Hasan Pathan1 and John Williams1,2
British Journal of Pain
6(1) 11–16
DOI: 10.1177/2049463712438493
……………………………………….
CNS Drugs. 2019 June ; 33(6): 567–580. doi:10.1007/s40263-019-00637-z.
Buprenorphine Treatment for Opioid Use Disorder: An Overview
Matisyahu Shulman, MD, Jonathan M. Wai, MD, and Edward V. Nunes, MD
………………………
Reward Processing by the Opioid System in the Brain
Julie Le Merrer, Jérôme A. J. Becker, Katia Befort, and Brigitte L. Kieffer
01 OCT 2009https://doi.org/10.1152/physrev.00005.2009
https://journals.physiology.org/pb-assets/images/physrev_logo-1598889796043.svg
https://journals.physiology.org/doi/full/10.1152/physrev.00005.2009
…………………
Distinct Effects of Nalmefene on Dopamine Uptake Rates and Kappa Opioid Receptor Activity in the Nucleus Accumbens Following Chronic Intermittent Ethanol Exposure
Sara R. Jones 1,*
Department of Physiology and Pharmacology Wake Forest School of Medicine, Winston-Salem, NC 27157, USA
H. Lundbeck A/S, Ottiliavej 9, 2500 Valby, Denmark
Int. J. Mol. Sci. 2016, 17(8), 1216; https://doi.org/10.3390/ijms17081216
https://www.mdpi.com/1422-0067/17/8/1216/htm
………………..
Extent and causes of global variations in access to morphine for medical use and actions to improve safe access
WHO
https://apps.who.int/iris/rest/bitstreams/1509689/retrieve
…………………………..
Untangling the complexity of opioid receptor function
- Rita J. Valentino & Nora D. Volkow
Published: 24 September 2018
https://www.nature.com/articles/s41386-018-0225-3/
……………….”….
PET imaging reveals lower kappa opioid receptor availability in alcoholics but no effect of age
- Aishwarya Vijay, Dana Cavallo, Alissa Goldberg, Bart de Laat, Nabeel Nabulsi, Yiyun Huang, Suchitra Krishnan-Sarin & Evan D. Morris
https://www.nature.com/articles/s41386-018-0199-1
…………………………………….
Clinical practice guideline for deprescribing opioid analgesics: summary of recommendations
Aili V Langford, Christine CW Lin, Lisa Bero, Fiona M Blyth, Jason Doctor, Simon Holliday, Yun‐Hee Jeon, Joanna Moullin, Bridin Murnion, Suzanne Nielsen, Rawa Osman, Jonathan Penm, Emily Reeve, Sharon Reid, Janet Wale, Carl R Schneider and Danijela Gnjidic
Med J Aust || doi: 10.5694/mja2.52002
Published online: 26 June 2023
………………………..
British Journal of Pharmacology (2006) 147, S153–S162
75 years of opioid research: the exciting but vain quest for the Holy Grail
1AlistairD.Corbett,2GraemeHenderson,*AlexanderT.McKnight&3StewartJ.Paterson
1Department of Biological & Biomedical Sciences, Glasgow Caledonian University, Glasgow G4 0BA; 2Department of Pharmacology, University of Bristol, University Walk, Bristol BS8 1TD and 3Kings College London, Department of Pharmacology and Therapeutics, GKT School of Biomedical & Health Sciences, Guy’s Campus, London Bridge, SE1 1UL
…………………………………………
February 5, 2020
Comparative Effectiveness of Different Treatment Pathways for Opioid Use Disorder
Sarah E. Wakeman, MD1,2; Marc R. Larochelle, MD, MPH3,4; Omid Ameli, MD, MPH5; et al
https://jamanetwork.com/journals/jamanetworkopen/article-abstract/2760032
JAMA NETWORK OPEN
………………………………….
Systemic κ-opioid receptor antagonism by nor-binaltorphimine reduces dependence-induced excessive alcohol self-administration in rats
Brendan M. Walker, Eric P. Zorrilla, George F. Koob
First published: 29 November 2010 https://doi.org/10.1111/j.1369-1600.2010.00226.xCitations: 151
https://onlinelibrary.wiley.com/doi/10.1111/j.1369-1600.2010.00226.x
…………………..
Cellular and Synaptic Adaptations Mediating Opioid Dependence
JOHN T. WILLIAMS, MACDONALD J. CHRISTIE, AND OLIVIER MANZONI
Vollum Institute, Oregon Health Sciences University, Portland, Oregon; Department of Pharmacology and The Medical Foundation, University of Sydney, Sydney, New South Wales, Australia; and Centre National de la Recherche Scientifique, Unite ́ de Propre de Recherche 9023, Montpellier, France
https://journals.physiology.org/doi/pdf/10.1152/physrev.2001.81.1.299
………………………………
Addiction. 2018 July ; 113(7): 1188–1209. doi:10.1111/add.14180.
Extended-release injectable naltrexone for opioid use disorder: A systematic review
Brantley P. Jarvis, PhD,
Department of Psychiatry and Behavioral Sciences, Johns Hopkins University School of Medicine
Public Health Research and Translational Science, Battelle Memorial Institute
August F. Holtyn, PhD,
Et Al.
…………………………
Journal of Substance Abuse Treatment
Volume 85, February 2018, Pages 49-55
Relapse to opioid use disorder after inpatient treatment: Protective effect of injection naltrexone
Edward V. Nunes a, Michael Gordon b, Peter D. Friedmann c, Marc J. Fishman d, Joshua D. Lee e, Donna T. Chen f, Mei Chen Hu g, Tamara Y. Boney i, Donna Wilson j, Charles P. O’Brien
https://www.sciencedirect.com/science/article/pii/S074054721630513X
……………………………………..
Addiction. 2013 February ; 108(2): 275–293. doi:10.1111/j.1360-0443.2012.04054.x.
Meta-analysis of naltrexone and acamprosate for treating alcohol use disorders: When are these medications most helpful?
Natalya C. Maisel1, Janet C. Blodgett1, Paula L. Wilbourne1, Keith Humphreys1,2, and John W. Finney1,2
1Center for Health Care Evaluation, VA Palo Alto Health Care System (152MPD), 795 Willow Rd., Menlo Park, CA 94025
2Stanford University Stanford School of Medicine, Department of Psychiatry & Behavioral Sciences, 401 N. Quarry Road, Stanford, CA 94305-5717
………………………………..

Leave a comment