ADDICTION PATHWAYS 2

This post takes a closer look at neuroadaptive changes occurring in the nucleus accumbens, the role of glutamine pathways, the amygdala, decreasing reward, and increasing negative pathological consequences as the disease progresses.
Some of the details of how this happens at the molecular level are now coming to light.
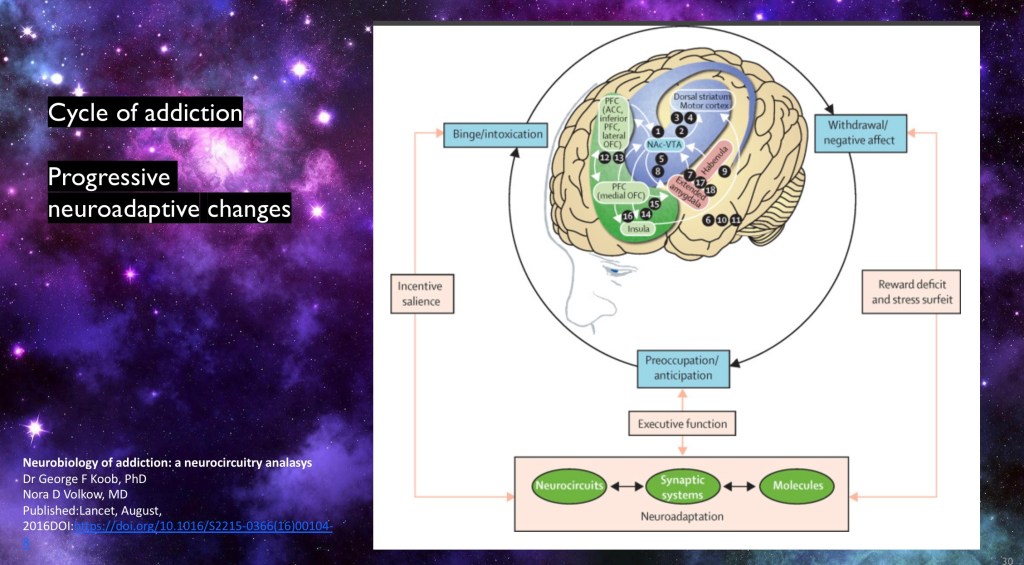
In this model positive affect / reward is followed by negative affect / anti reward. Over time positive rewarding effects diminish and reward deficit and stress effects increase. These may include hangovers and withdrawal symptoms. The individual becomes preoccupied with the next rewarding experience.
Research is finding some of the key biological functions underlying this cycle at the cellular and neural circuit level.
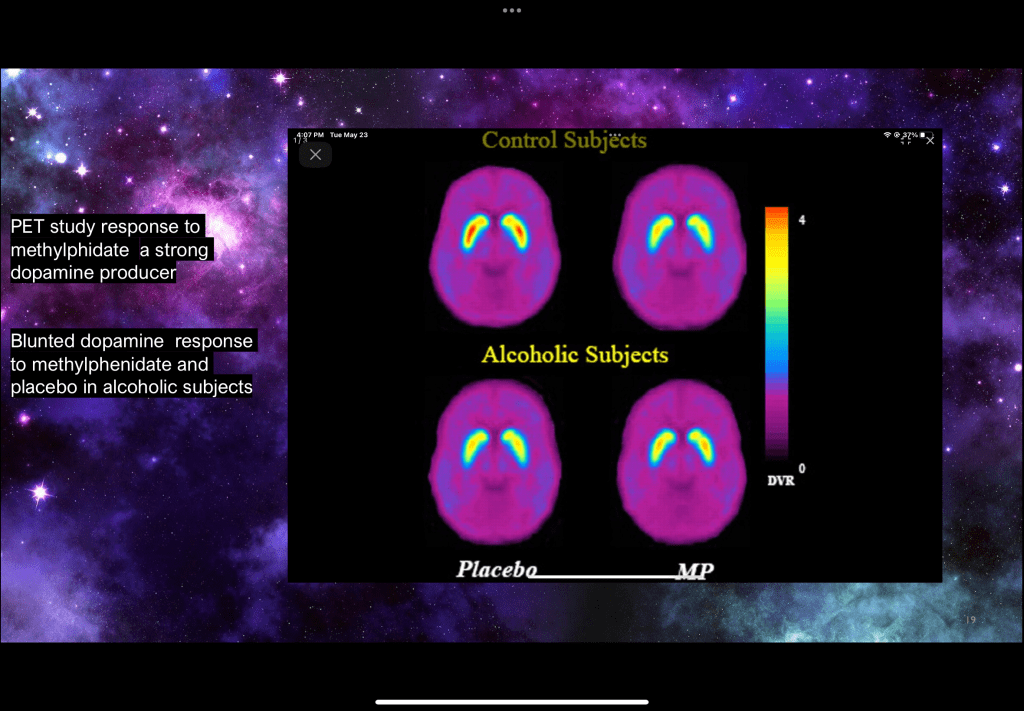
This PET study labeled dopamine receptor availability in response to placebo and IV methylphenidate. The areas with activity are in the dorsal striatum. Alcohol addicted subjects demonstrate decreased receptor labeling with both placebo and Ritalin dose. Control subjects have more receptors with placebo. After the drug dose in controls many receptors are occupied by dopamine so fewer receptors are available for labeling.
This provides strong evidence for down regulation of the dopamine reward pathway in addicted individuals with and without the addictive substance present. There are fewer receptors present without the drug and the dopamine response to a rewarding stimulus is blunted.
This explains why in active addiction larger and more frequent doses are sought. Often people lose interest in activities they formerly enjoyed. It also explains why in abstinence, particularly early on, people feel down or depressed. The reward pathway is damaged and takes time to repair.
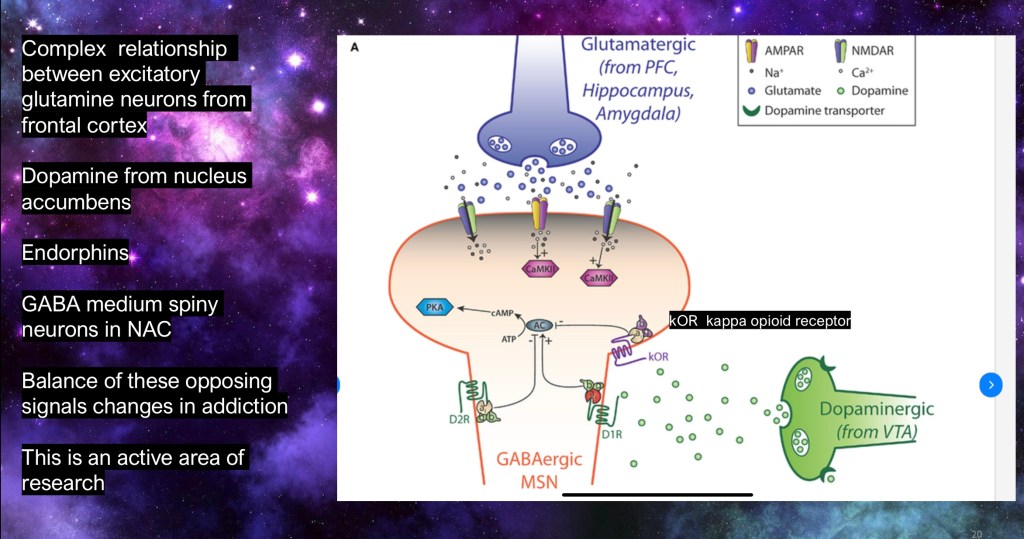
The diagram combines some of the neural inputs we have been looking at. This represents inputs to medium spiny neurons located in the nucleus accumbens. Most of these produce inhibitory GABA neurotransmitter.
In green is a neural dopamine synapse coming up from the VTA in the midbrain.
In blue is an excitatory glutamate input coming in from the frontal cortex synapsing with the same neuron. Under normal circumstances these are in delicate balance and the neuron responds appropriately.
Also shown is an endorphin receptor influencing the same neuron. These also contribute to normal signaling.
Because of changes in input from these neurotransmitters the medium spiny neurons no longer respond appropriately.
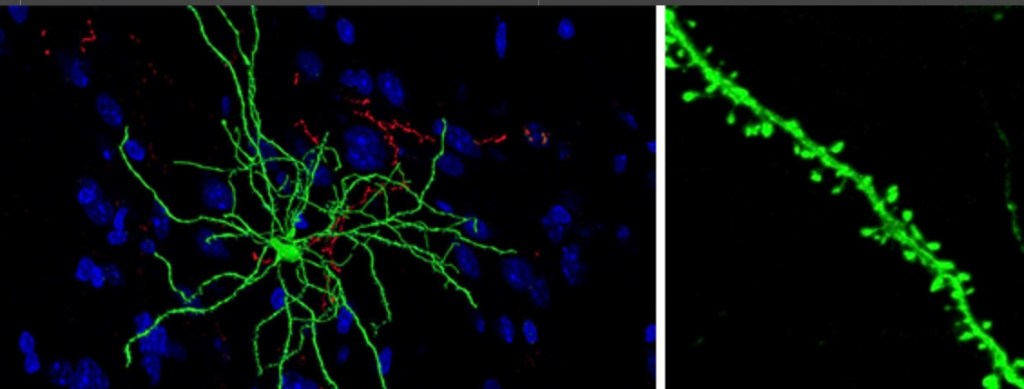
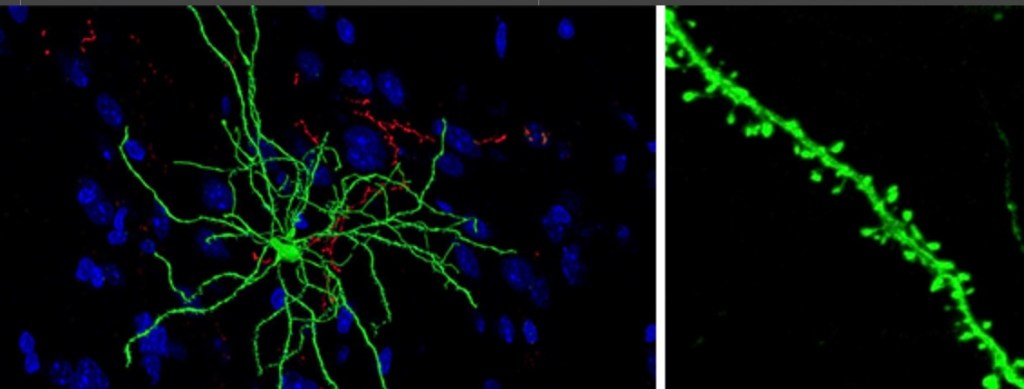
The next few studies demonstrate some of the physical changes occurring in these regulatory inter neurons in the NAC. These are medium spiny neurons and they are…spiny.
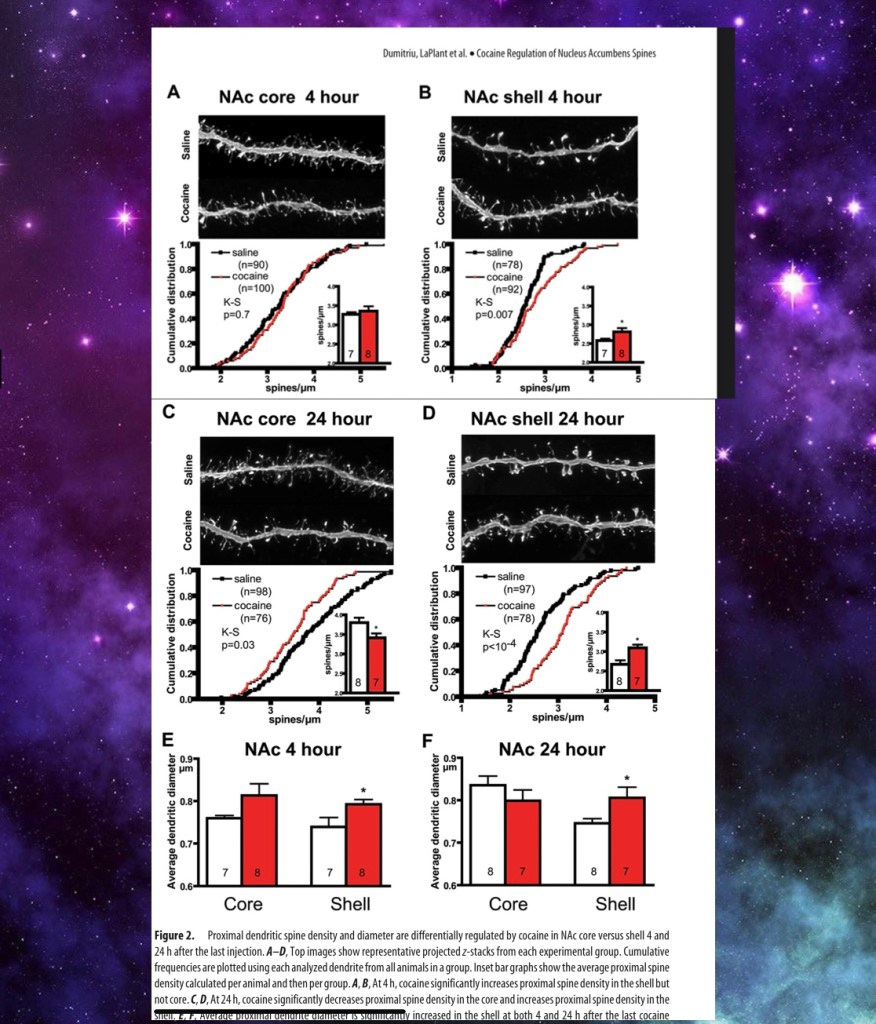
This study looked at neuroplastic structural changes occurring in medium spiny neurons (MSN). Red = cocaine white = saline
Dendrites are shown. There are these tiny spines you can see projecting along the dendrites. Changes in the number of these little spines have been noted in addiction which would affect the sensitivity of the cells. Here white bars represent saline control and red bars density of spines following cocaine administration at 4 and 24 hours.
Shell and core are different regions of the NAC. One day after a dose of cocaine there are changes in spine density in the core and shell. This changes neural sensitivity and is thought to further contribute to signaling imbalance. This is hypothesized to be involved in learning.
These neuroplastic changes have been shown to be long lasting and are thought to contribute to relapse.
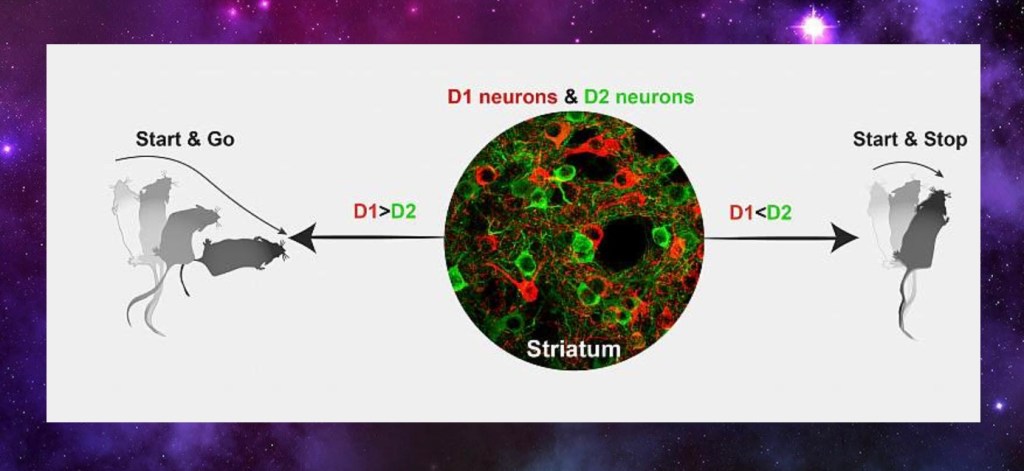
There are two types of dopamine receptors, D1 and D2. These differ in action. If D1 receptors dominate the response is more excitatory, leading to increased activity.
If D2 receptors dominate there is less behavioral activity. Receptor activity relates to motivation.
Under normal conditions there is a balance of these receptor types. In addiction there is an increased excitatory D1 predominance. This generates a stronger response and requires a stronger stimulus to activate.
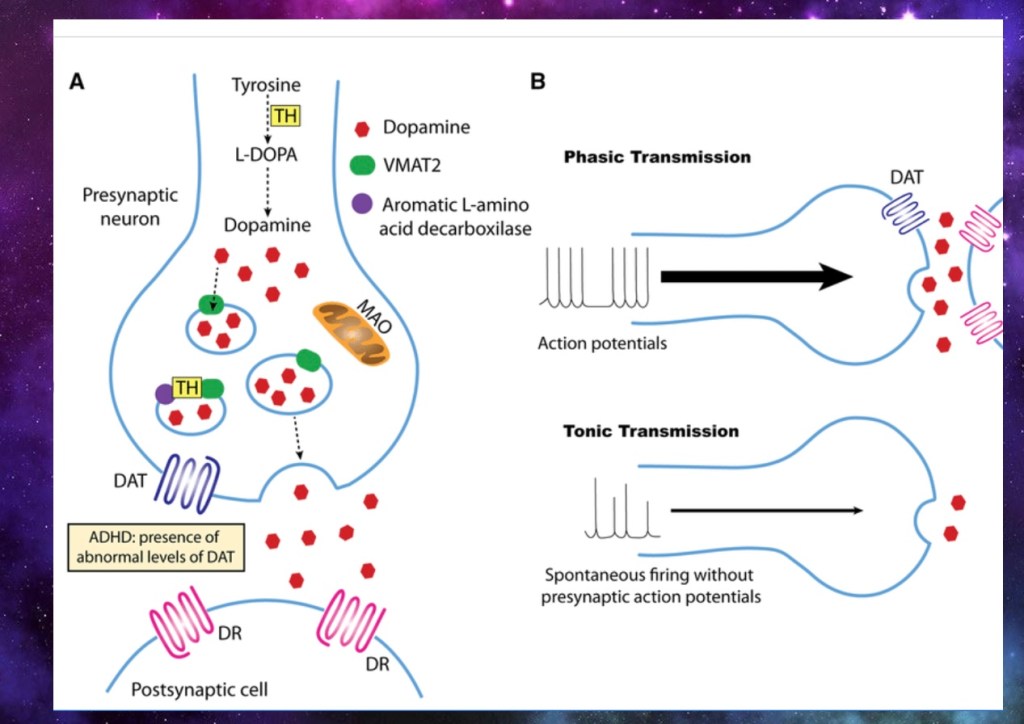
Phasic firing releases a burst of neurotransmitter resulting in a more powerful short signal. D1 receptors respond to this type of firing.
Tonic release does not require an action potential and releases a slow steady state of neurotransmitter. D2 receptors are more sensitive to tonic release.
In addiction D1 receptors predominate. This is a neuroplastic change in DNA and RNA activating the receiving cell to manufacture more D1 receptors. Stronger signals are required to activate the rewarding circuitry as addiction progresses.
To get some perspective on what this all means in actual experience.
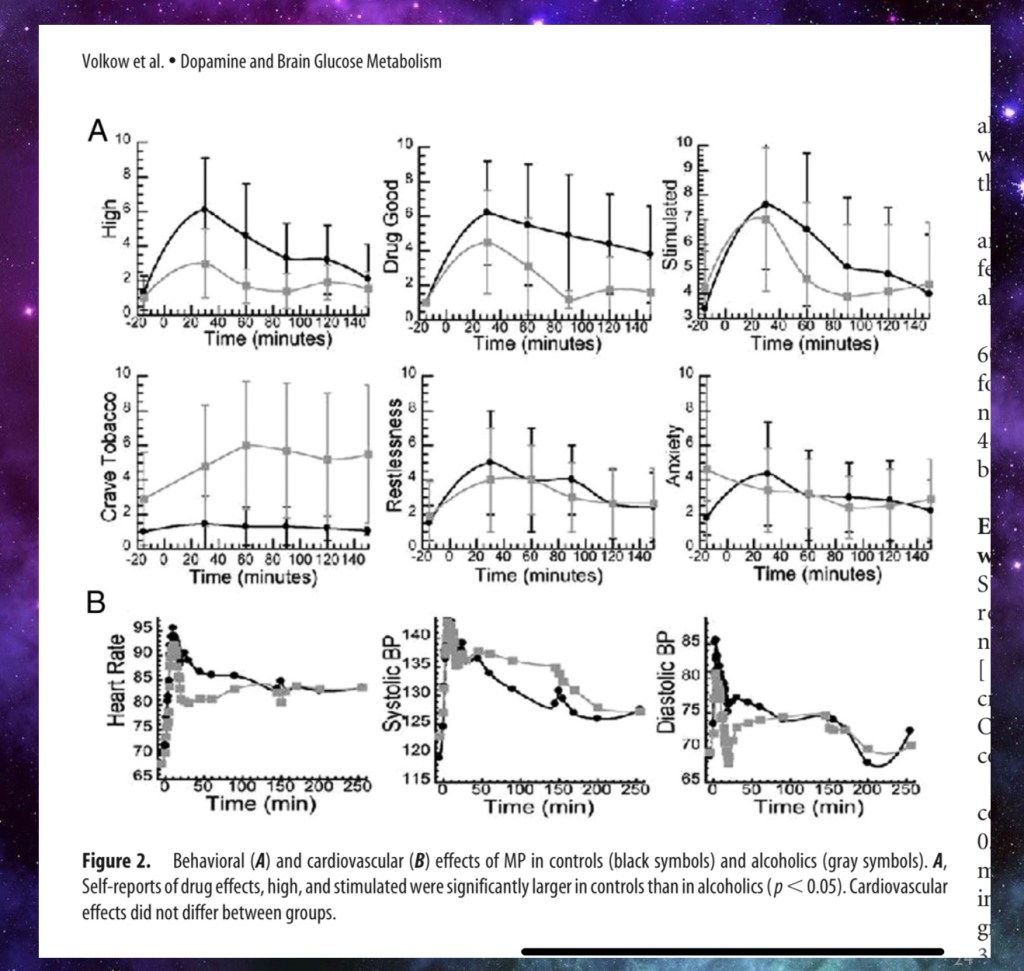
Grey = addicted Black = controls
Here they gave controls and alcohol addicted subjects IV Ritalin and had each group rate subjective feelings: how “high”, “good” or “stimulated” they felt. Alcohol addicted subjects rated these lower. The same dose resulted in less reward for the addicted group. They reported increased tobacco desire compared to controls. The two groups felt about the same for “restlessness” and “anxiety”. These feelings result from different pathways.
Note that objective measurement of blood pressure and heart rate were the same for both groups.
As regular drug use continues it becomes less and less rewarding. Increased dose is required to maintain what has become a normal state.
A closer look at what is going on in the frontal lobe.
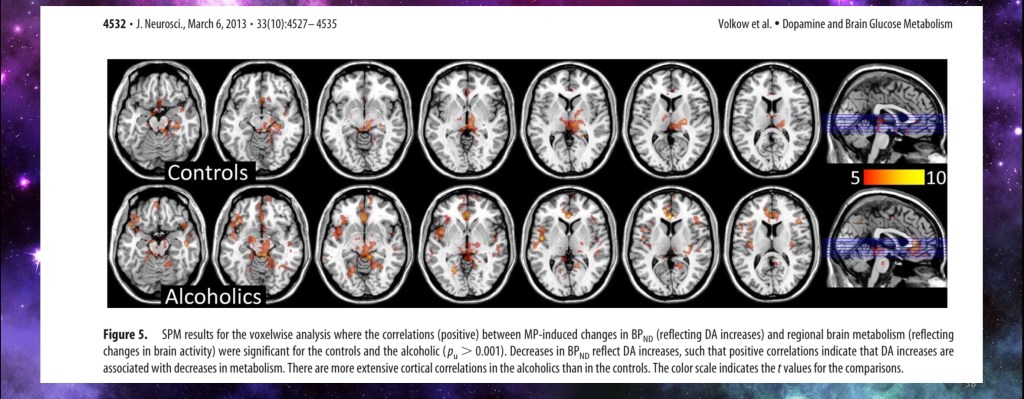
This is a PET study combining changes reflecting local dopamine increases and glucose metabolism. Alcohol addiction compared to controls following Ritalin injection.
This series shows areas with both local dopamine increased activity and metabolic decreased activity. In alcohol addiction there were more cortical areas with this finding than in controls.
This indicates that the cortex in addiction is less active overall yet more influenced by addictive drug than in control subjects. As addiction progresses motivation and drive becomes increasingly narrowed. What was a choice becomes a necessity. What was a thought becomes an obsession.
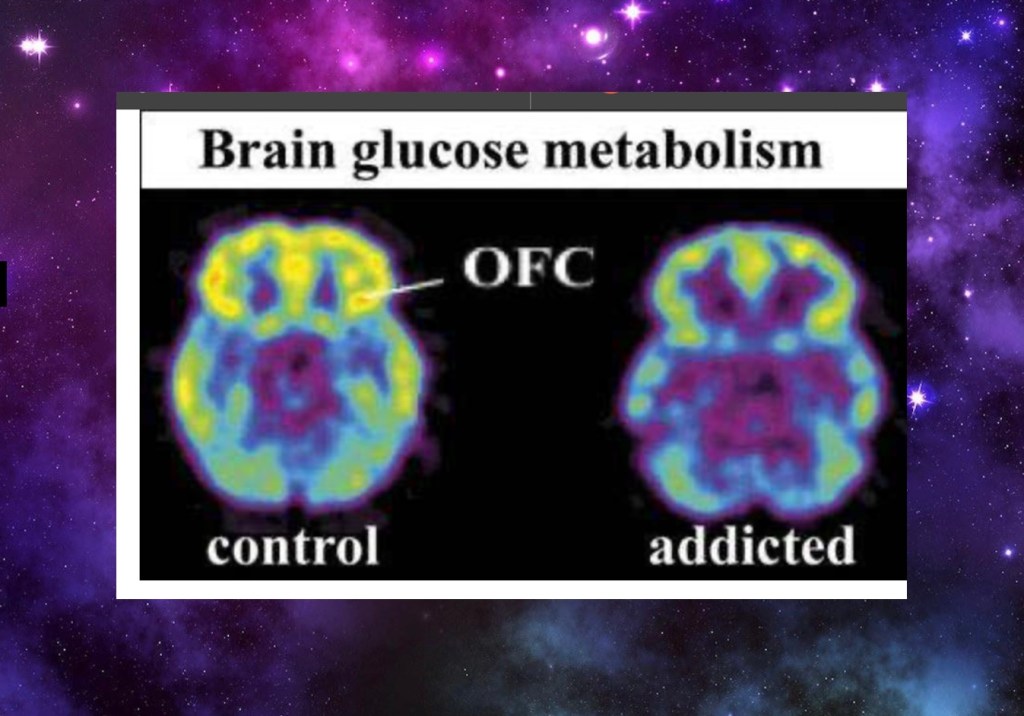
PET study demonstrating overall decreased frontal cortical metabolism at baseline in addicted subjects. Decreased activity is also seen in the temporal lobe insular cortex.
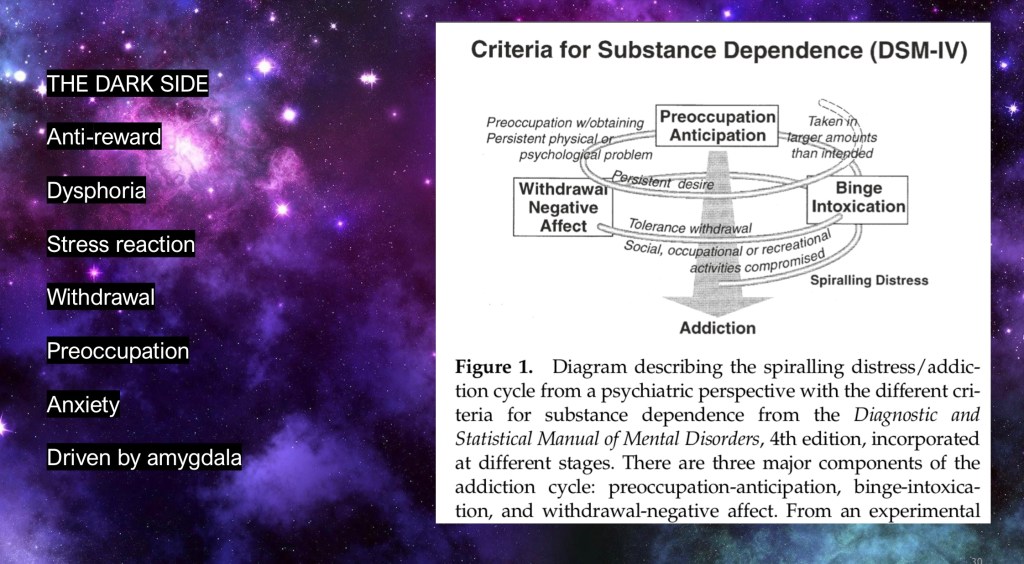
This model comes from the work of George Koob who has been studying the “dark side” of addiction for decades. This focuses on the role of the amygdala and related stress reactions occurring in active addiction. Rather than seeking positive reward, use increasingly becomes a drive to avoid negative consequences.
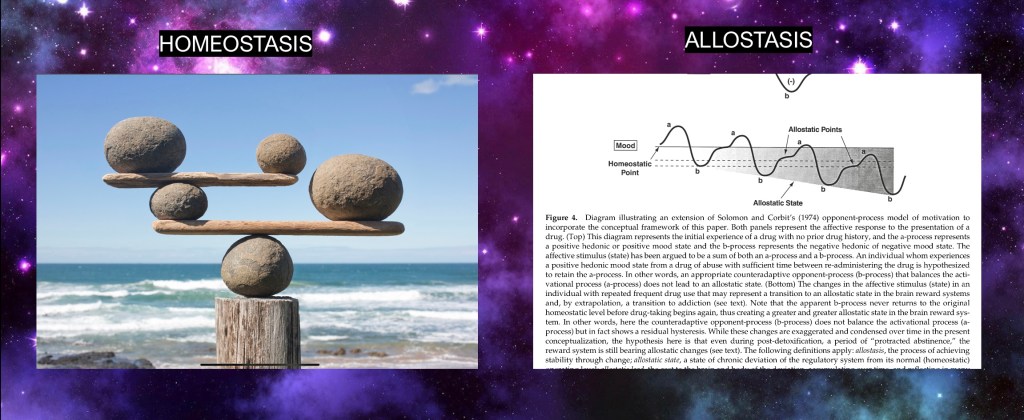
Fundamental to any biological system is maintaining homeostasis. Regulatory systems act to maintain balance around optimal parameters. This is constantly happening on a subconscious level in response to changing environmental conditions.
The stress response is recruited in the addictive process. As we have seen imbalances in the dopaminergic reward system result in maladaptive changes in regulatory inter neurons and frontal cortical function. To add to this negative reactions kick in further driving the addictive cycle.
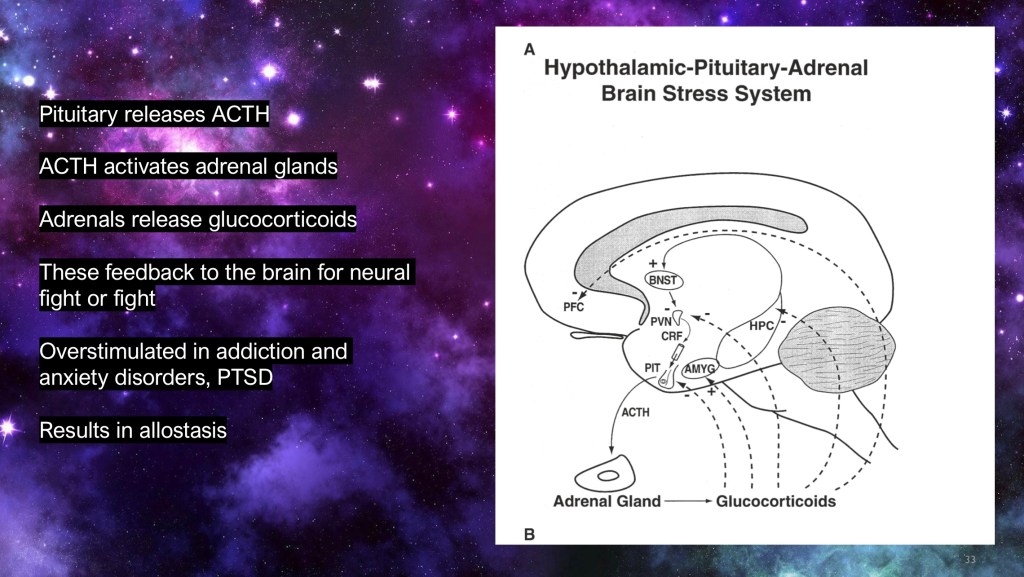
Activation of the hypothalamic pituitary axis results in release of ACTH from the pituitary gland and glucocorticoids from the adrenals. These have effects throughout the body as well as the brain preparing it for fight or flight.
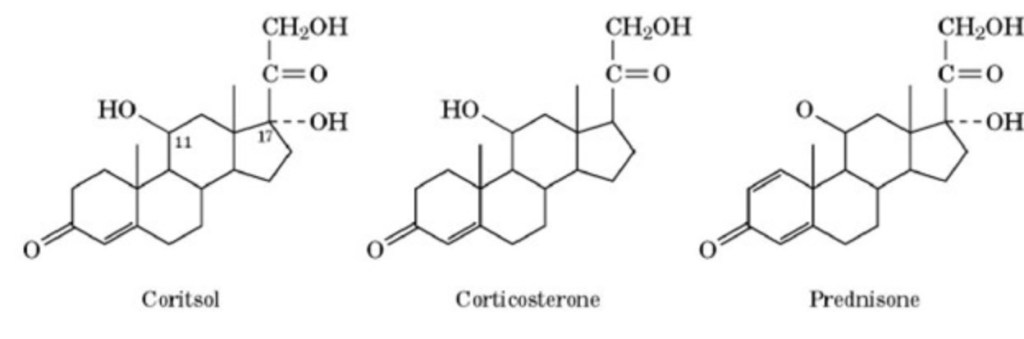
Glucocorticoids
In addiction there is increased activation of this pathway which becomes disinhibited due to imbalance of signals coming in from the NAC, cortex and VTA.
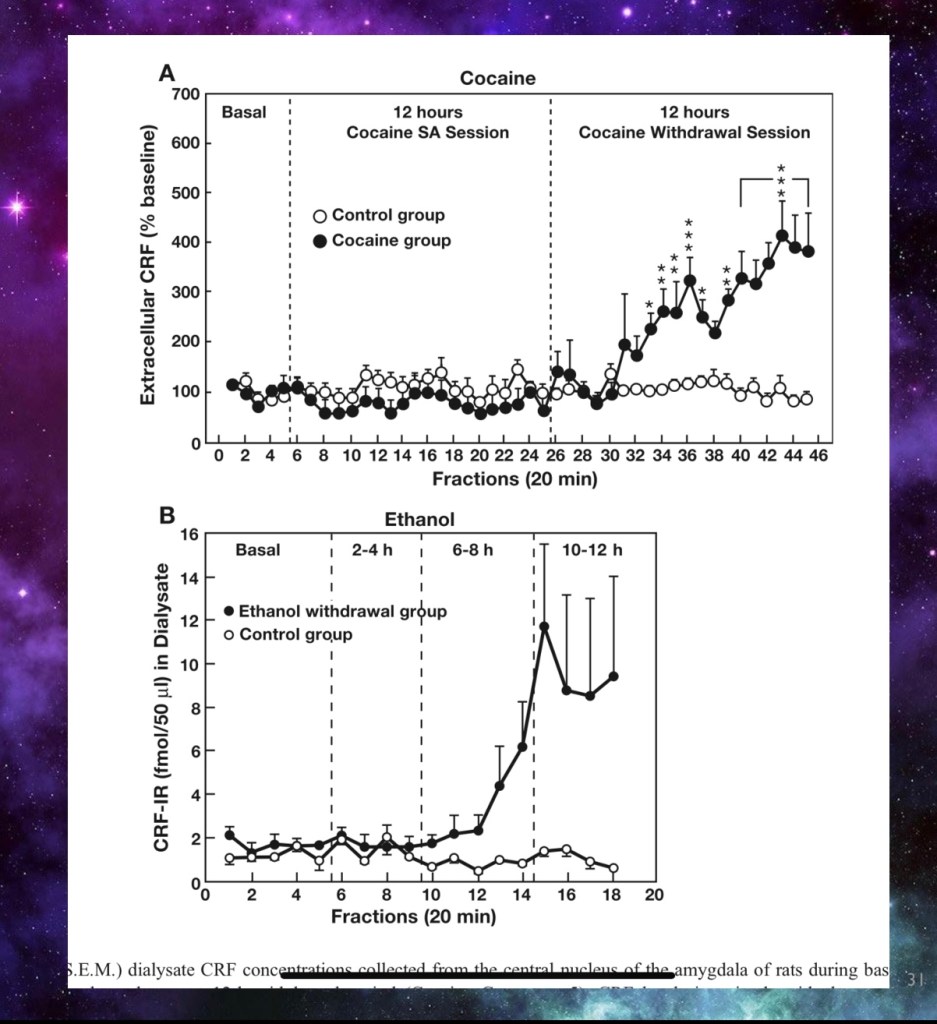
Corticotropin releasing factor was measured after discontinuation of:
- A) cocaine
- B) ethanol.
- Both the extra pituitary and pituitary mediated stress pathways are activated by CRF when the drug is discontinued.
In addition to activating reward pathways addictive drugs tend to have the property of masking or suppressing dysphoric stress related symptoms further driving the addiction cycle.
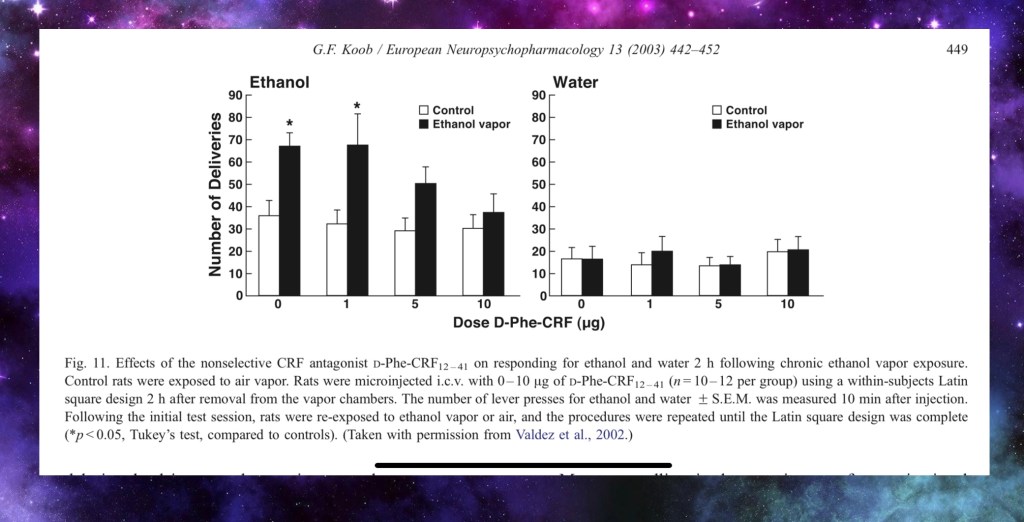
In this experiment rats were chronically exposed to ethanol vapor. These are compared to a control group. Both groups were given an agent that blocks activity of CRF at increasing doses ( D-Phe-CRF).
Blocking CRF activity results in fewer alcohol bottle lever presses in a dose dependent response. This is not seen in non dependent rats.
Both groups were the same when offered only water as an additional control.
This indicates a direct dose – response to alcohol seeking behavior due to Corticotropin releasing factor induced by alcohol dependency.
Additional factors have been implicated in addiction. The endorphin endogenous opioid system has been shown to have a role. There is also some evidence for involvement of the endocannabinoid system. These findings would be an interesting topic for additional review.

Thank you for your consideration in reading this post. Any feedback is appreciated.
For information and educational purposes only. No commercial or institutional interests. This post should not be considered medical or professional advice.
REFERENCES
Loss of Dopamine Transporters in Methamphetamine Abusers Recovers with Protracted Abstinence
Nora D. Volkow, Linda Chang, Gene-Jack Wang, Joanna S. Fowler, Dinko Franceschi, Mark Sedler, Samuel J. Gatley, Eric Miller, Robert Hitzemann, Yu-Shin Ding and Jean Logan
Journal of Neuroscience 1 December 2001, 21 (23
https://www.jneurosci.org/content/21/23/9414
……………………….
Behavioral/Systems/Cognitive
Nucleus Accumbens Medium Spiny Neurons Target Non-Dopaminergic Neurons in the Ventral Tegmental Area
Yanfang Xia, Joseph R. Driscoll, Linda Wilbrecht, Elyssa B. Margolis, Howard L. Fields, and Gregory O. Hjelmstad
Ernest Gallo Clinic and Research Center, Wheeler Center for the Neurobiology of Addiction, and Department of Neurology, University of California, San Francisco, Emeryville, California 94608
The Journal of Neuroscience, May 25, 2011 • 31(21):7811–7816 • 7811
…………………….
Effects of alcohol on the membrane excitability and synaptic transmission of medium spiny neurons in the nucleus accumbens
Author links open overlay panel
Vincent N. Marty, Igor Spigelman
Volume 46, Issue 4, June 2012, Pages 317-327
https://www.sciencedirect.com/science/article/abs/pii/S0741832911005234
………………………
Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications
Nat Rev Neurosci. Author manuscript; available in PMC 2012 Oct 2.Published in final edited form as:Nat Rev Neurosci. 2011 Oct 20; 12(11): 652–669. Published online 2011 Oct 20. doi: 10.1038/nrn3119
PMCID: PMC3462342NIHMSID: NIHMS408806PMID
Volkow NM
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3462342/
………..
The contribution of medium spiny neuron subtypes in the nucleus accumbens core to compulsive-like ethanol drinking
Author links open overlay panel
Elizabeth A. Sneddon, Kristen M. Schuh, John W. Frankel, Anna K. Radke
Volume 187, 1 April 2021, 108497
……………………….
Drug Addiction, Dysregulation of Reward,
and Allostasis
George F. Koob, Ph.D., and Michel Le Moal, M.D., Ph.D.
…………………………
- Published: 26 August 2009
Neurocircuitry of Addiction
- George F Koob & Nora D Volkow
volume
35,
pages
217–238 (2010)Cite this article
……………………………
Profound Decreases in Dopamine Release in Striatum in Detoxified Alcoholics: Possible Orbitofrontal Involvement
Nora D. Volkow, Gene-Jack Wang, Frank Telang, Joanna S. Fowler, Jean Logan, Millard Jayne, Yeming Ma, Kith Pradhan and Christopher Wong
Journal of Neuroscience 14 November 2007, 27 (46
https://www.jneurosci.org/content/27/46/12700/tab-figures-data
……………………………….
PET imaging of dopamine transporter and drug craving during methadone maintenance treatment and after prolonged abstinence in heroin users
Author links open overlay panel
Jie Shi a, Li-Yan Zhao a, Marc L. Copersino b, Yu-Xia Fang c, Yingmao Chen d, Jiahe Tian d, Yanping Deng a, Yinliang Shuai e, Jun Jin e, Lin
https://www.sciencedirect.com/science/article/abs/pii/S0014299907010953
…………………………….
Eur J Neurosci. 2009 September ; 30(6): 1117–1127. doi:10.1111/j.1460-9568.2009.06916.x.
Neural Encoding of Cocaine Seeking Behavior is Coincident with Phasic Dopamine Release in the Accumbens Core and Shell
Catarina A. Owesson-White1, Jennifer Ariansen2, Garret D. Stuber3, Nathan A. Cleaveland1, Joseph F. Cheer2, R. Mark Wightman2,3,4, and Regina M. Carelli1,3,4
https://cdr.lib.unc.edu/downloads/44558m38n
…………………………….
Addiction: Beyond dopamine reward circuitry
Nora D. Volkow nvolkow@nida.nih.gov, Gene-Jack Wang, Joanna S. Fowler, +1 , and Frank TelangAuthors Info & Affiliations
Edited by Donald W. Pfaff, The Rockefeller University, New York, NY, and approved November 9, 2010 (received for review August 31, 2010)
March 14, 2011
https://www.pnas.org/doi/10.1073/pnas.1010654108
………………………………
Neurotransmitter and neuromodulatory mechanisms involved in alcohol abuse and alcoholism
Author links open overlay panel
Igal Nevo, Michel Hamon
Volume 26, Issue 4, April 1995, Pages 305-336
………………………………
Activation of orbital and medial prefrontal cortex by methylphenidate in cocaine-addicted subjects but not in controls: relevance to addiction
Nora D Volkow 1 , Gene-Jack Wang, Yeming Ma, Joanna S Fowler, Christopher Wong, Yu-Shin Ding, Robert Hitzemann, James M Swanson, Peter Kalivas
Affiliations expand
2005 Apr 13;25(15):3932-9. doi: 10.1523/JNEUROSCI.0433-05.2005.
https://pubmed.ncbi.nlm.nih.gov/15829645/
……………………………….
Neuroscience. 2014 September 26; 0: 139–151. doi:10.1016/j.neuroscience.2014.06.053.
Alcohol, stress hormones, and the prefrontal cortex: a proposed pathway to the dark side of addiction
Yi-Ling Lua and Heather N. Richardsonb,*
aNeuroscience and Behavior Program, University of Massachusetts, Amherst, MA 01003, United States
bDepartment of Psychological and Brain Sciences, University of Massachusetts, Amherst, MA 01003, United States
https://www.sciencedirect.com/science/article/abs/pii/S0306452214005387
……………………………
Biol Psychiatry. 2015 May 15; 77(10): 859–869. doi:10.1016/j.biopsych.2014.09.008.
The Central Amygdala as an Integrative Hub for Anxiety and Alcohol Use Disorders
Nicholas W. Gilpin1,2, Melissa A. Herman3, and Marisa Roberto3
1Department of Physiology, Louisiana State University Health Sciences Center, New Orleans, LA
2Neuroscience Center of Excellence, Louisiana State University Health Sciences Center, New Orleans, LA
3Committee on the Neurobiology of Addictive Disorders, The Scripps Research Institute, La Jolla, CA

Leave a comment