Astrocytes and glutamate: role in addiction and relapse
Over the past 30 years there has been rapid advancement in understanding the neurobiological basis of substance addiction. Progressive loss of control and relapse are characteristic in substance use disorder. There are unanswered questions concerning relapse at the cellular level.
This post reviews the glutamate homeostasis hypothesis explaining a key mechanism responsible for continued use in active addiction and relapse.
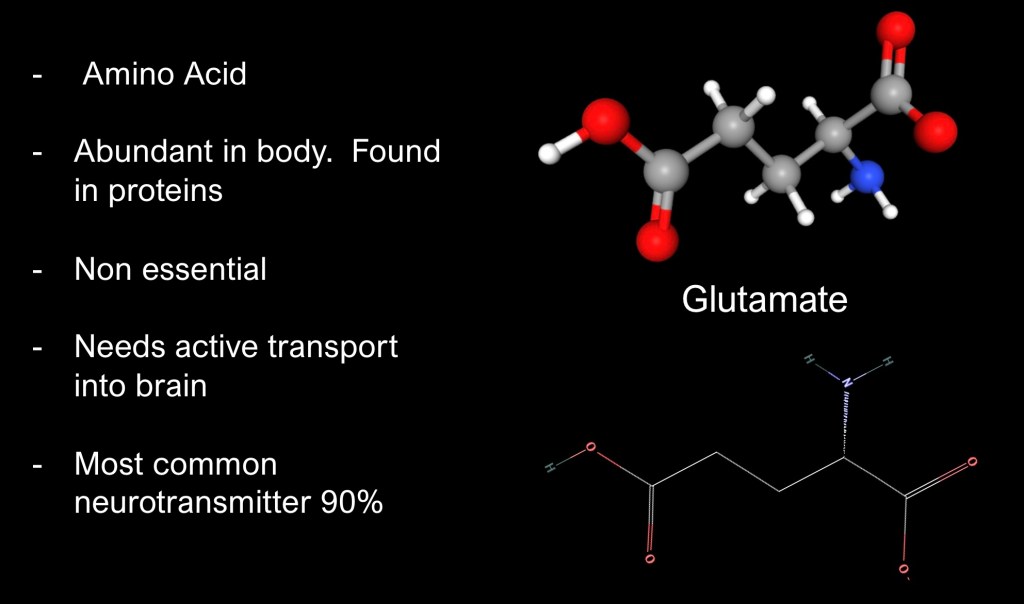
Glutamate is an amino acid found in many dietary sources and abundant in the human body. It can be synthesized from precursors and is considered a non essential amino acid.
It is the most common neurotransmitter in the central nervous system comprising about 90% of synapses. Glutamate is an excitatory neurotransmitter and diffusely distributed throughout the brain.
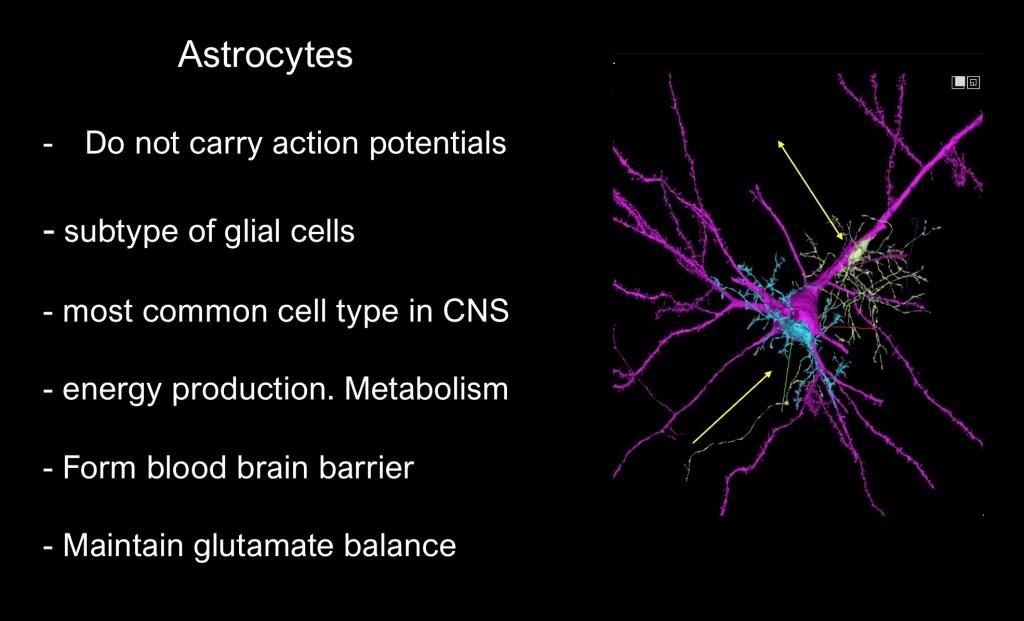
Astrocytes are a subset of glial cells and are the most abundant cell type in the brain. Unlike neurons they do not carry action potentials. Astrocytes surround cerebral blood vessels and have an essential role in maintaining the blood brain barrier. They function in metabolic and immune support roles.
Astrocytes play a central role in glutamate transmission. Changes in the ability of synaptic astrocytes to regulate glutamate levels in chronic substance use are central mechanisms in addiction and relapse.
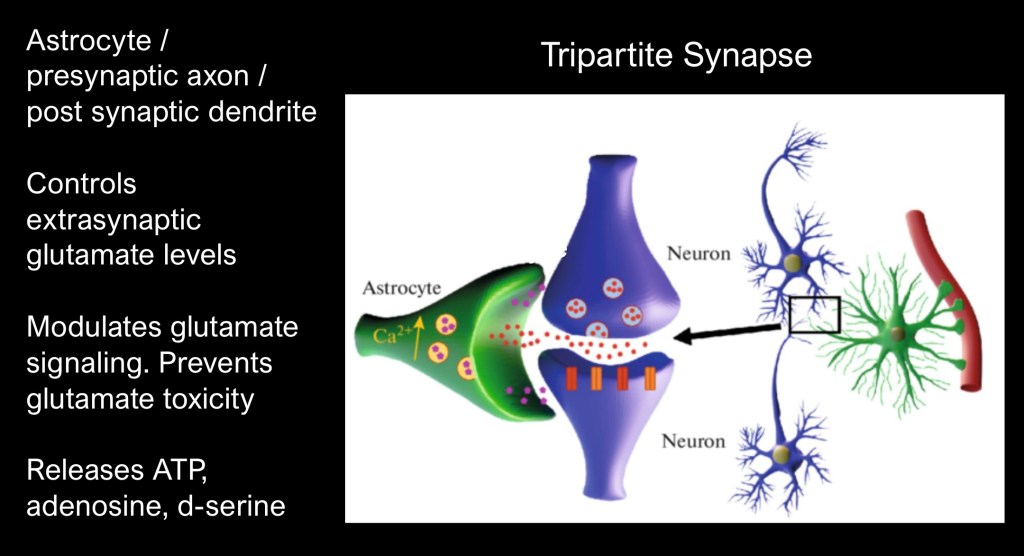
The tripartite synapse is a functional unit composed of a presynaptic axon, post synaptic dendrite, and an astrocyte which wraps around the synaptic space.
Glutamate levels are tightly regulated within the tripartite synapse. Presynaptic release is controlled by extrasynaptic receptors, metabotropic and ionotropic post synaptic receptors, astrocytic transporters and glutamate cysteine exchangers.
Glutamate transmission consists of a flow involving fluctuating synaptic and extrasynaptic neurotransmitter levels maintained in a complex system of checks and balances. Given the centrality of glutamate in brain function small deviations may have major impact. Glutamate imbalance is a key component in substance addiction and relapse.
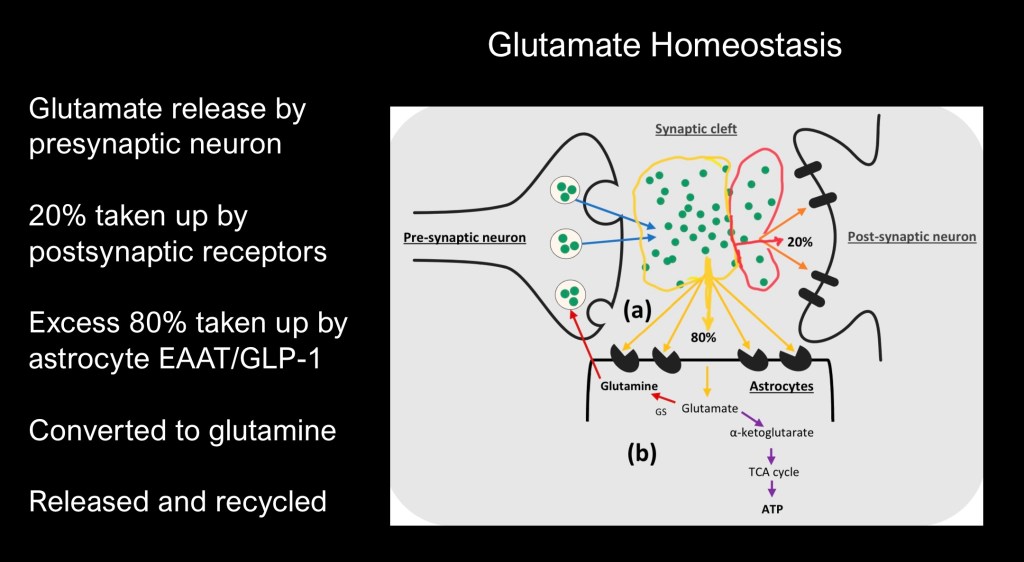
When a glutaminergic neuron releases into the synaptic space only 20% is taken up by postsynaptic receptors. The remainder spills over into the extrasynaptic space and most is taken up by astrocytes. Some of this is metabolized into αketogluterate and processed through the TCA cycle into energy in the form of ATP.
The remainder is converted into glutamine which can be taken up by the presynaptic neuron and recycled back into glutamate to be stored in vesicles for the next transmission.
In homeostasis overall glutamate tone is regulated to transmit appropriately.
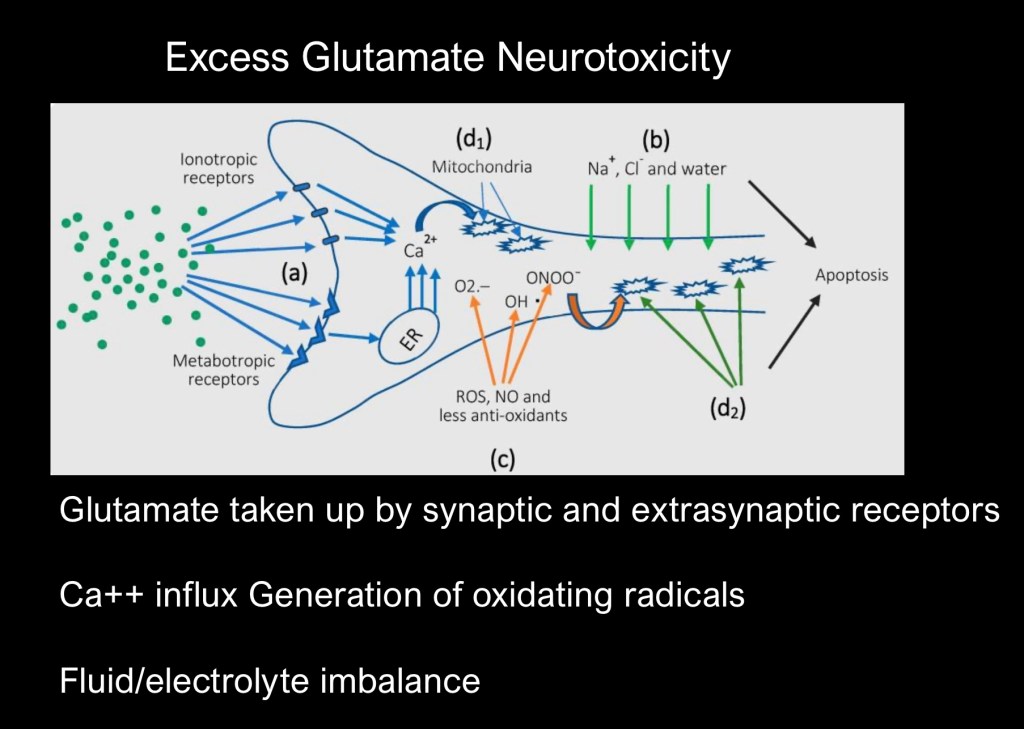
If synaptic levels are too high neuronal toxicity may occur. Excess excitatory transmission releases calcium stores within the cell. This results in mitochondrial damage and generation of oxidizing radicals. Excess water and ionic flow into the cell can result in apoptosis and cell death.
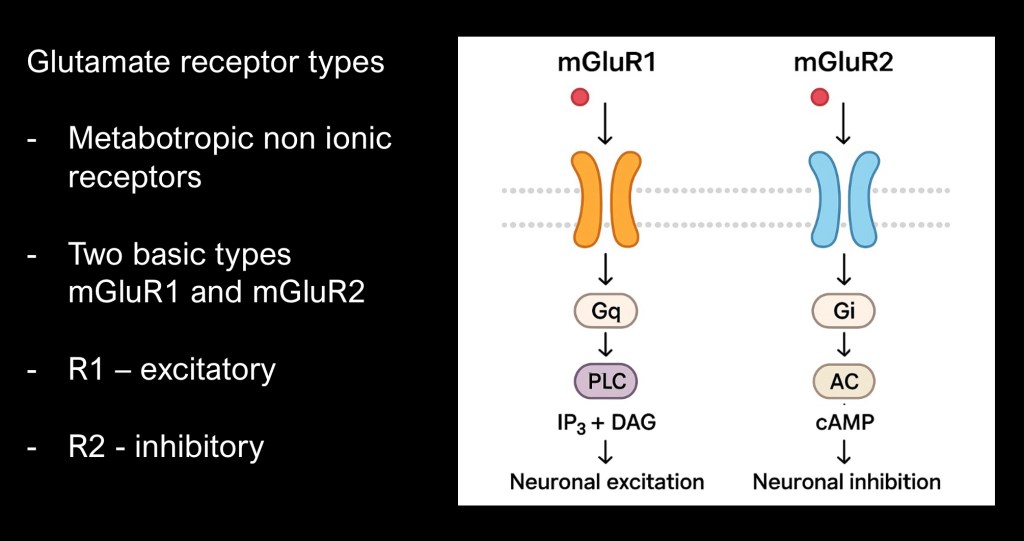
There are eight known glutamate receptor subtypes. These may be grouped into two classes. Group one (mGluR1) are mostly within the synapse and promote neuronal excitability.
Group 2 (mGluR2, or 2/3) are presynaptic and are inhibitory. Because they are outside the synapse they take slightly longer to act. They serve as an “off switch” for the signal.
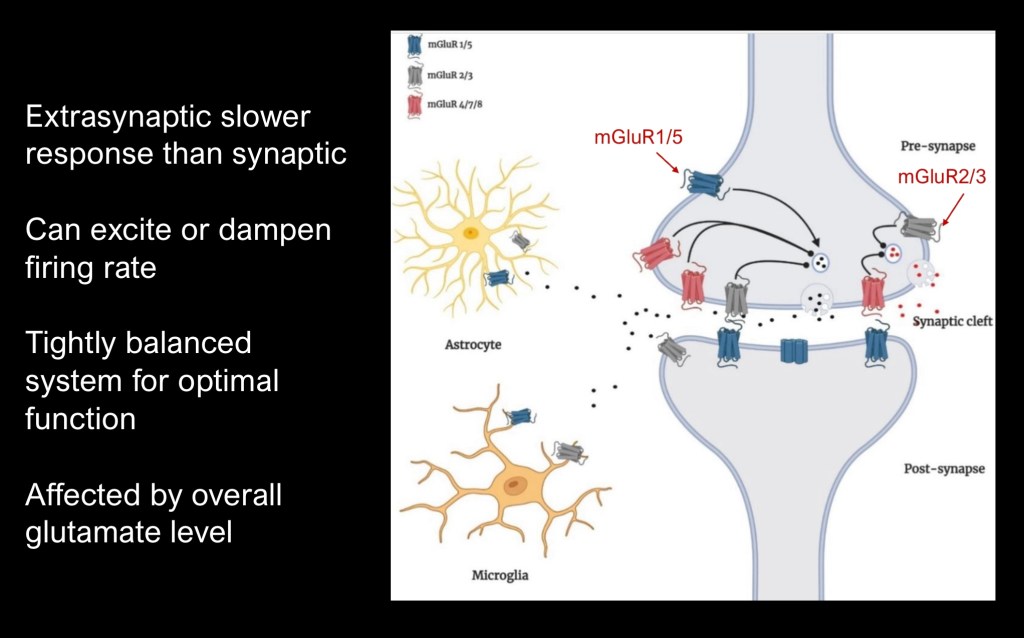
Diagram depicting mGluR2/3 on the upper synaptic margin of the cell where it would respond to excess glutamate by shutting down transmission.
Receptors also may be present on adjacent astrocytes and microglia.
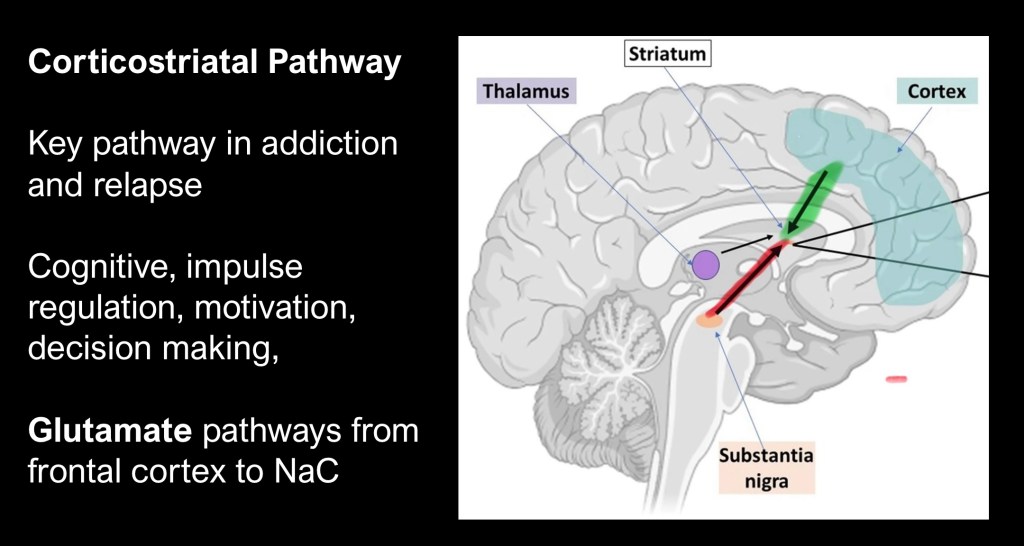
The neural pathway shown in green above is compromised in addiction. The frontal-striatal glutaminergic pathway extends from the medial frontal cortex to the Nucleus Accumbens (NAc) and dorsal striatum. Shown in red is the dopaminergic reward pathway converging on the same structures.
Preclinical studies have demonstrated a central role for the medial prefrontal cortex to nucleus accumbens pathway in reinstatement of drug seeking behavior in animal models. Inactivation of neurons at the cortical or accumbens eliminates drug seeking and reactivation restores relapse behavior.
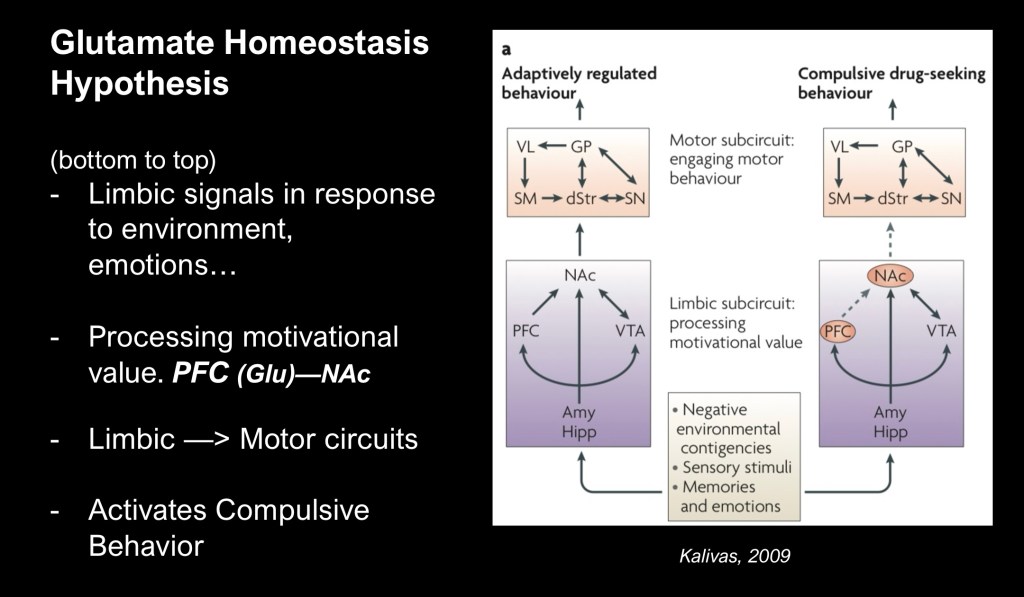
Peter Kalivas at Medical University of South Carolina has been one of the principal investigators into the role of unbalanced glutamate in addiction and relapse.
This model illustrates factors common in initial drug use such as emotional stressors and environmental stimuli. At the base are involved limbic responses and the mesolimbic dopamine reward system. Adaptive responses diverge at the level of cognitive discriminative input from the frontal cortex to the NAc resulting in maladaptive signals to motor areas of the brain. This results in addictive behavior.

A closer look at glutamate homeostasis and how that is affected in addiction.
At the bottom of the diagram highlighted in green are astrocyte glutamate transporters and cysteine – glutamate exchangers. These result in uptake of excess glutamate by astrocytes.
At the synaptic margins of pre and post synaptic neurons metabotropic glutamate receptors mGluR2/3 and mGluR5 which in the presence of perisynaptic excess glutamate will shut down transmission and increase excitability of the receiving neuron.
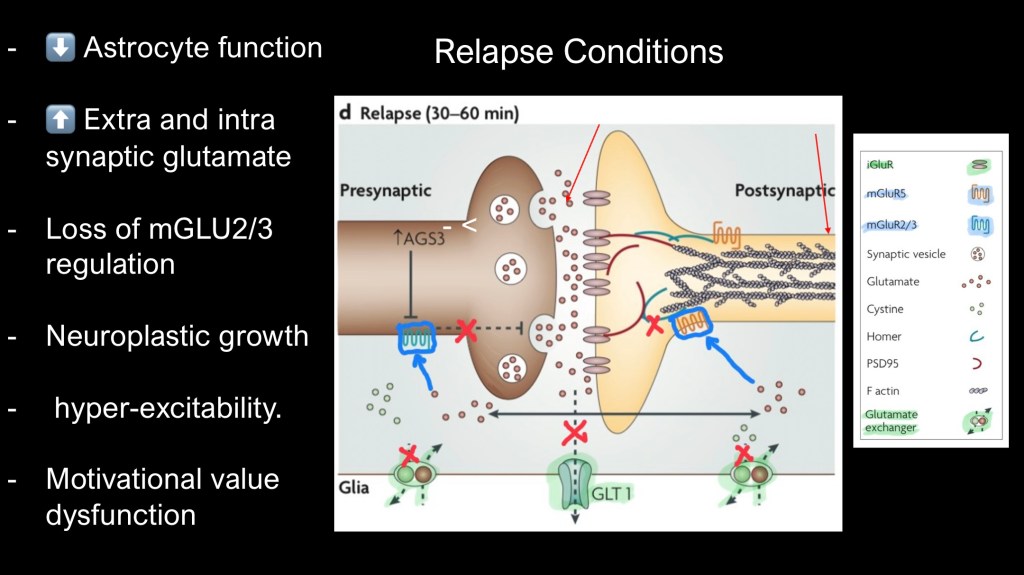
What happens in active addiction. The diagram highlights long term neuroplastic changes in compulsive drug use. Glutamate levels within the synapse are increased and perisynaptic levels are decreased.
Astrocytes have impaired uptake and exchange of glutamate. With lower perisynaptic levels mGluR2/3 receptors do not effectively “turn off” the flow of glutamate.
This results in hyperexcitability of receiving neurons now expressing more ionotropic receptors along with morphological changes.
Internally cytoskeletal F actin is recruited and more, larger receptive dendrites and synapses grow. This more sensitized neuroplastic physical change is long term and a major driver of relapse behavior. It means that the threshold of internal and external stimuli needed for initiation of drug seeking behavior is much lower. This accompanies diminished cognitive control over consumption.
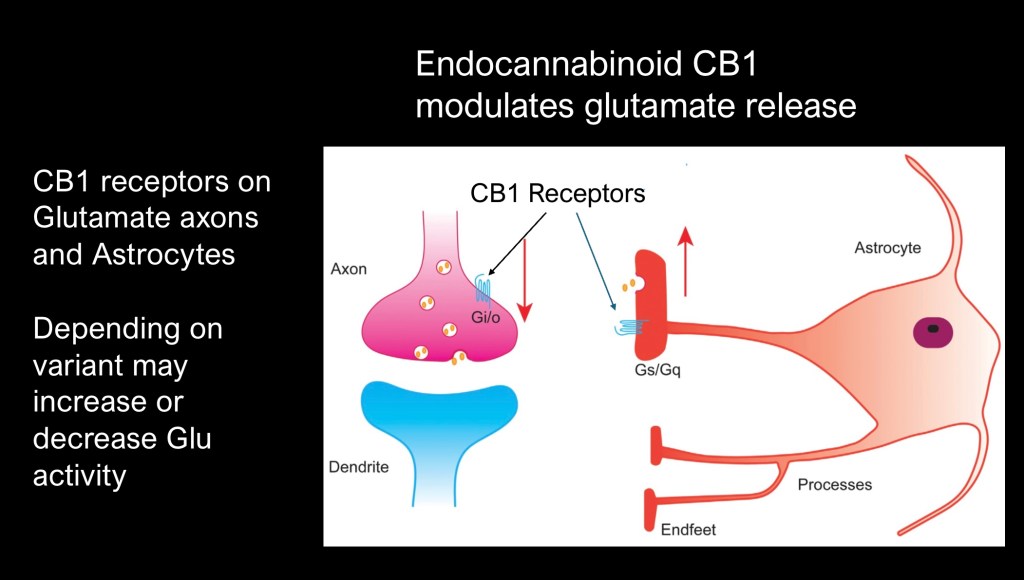
There is a growing body of evidence for a regulatory role played by the endocannabinoid (EC) system in glutamate homeostasis. CB1 receptors are present in synaptic axonal terminals and astrocyte end feet. Activation by endocannabinoids may either promote or inhibit neural activity depending on the G protein involved.
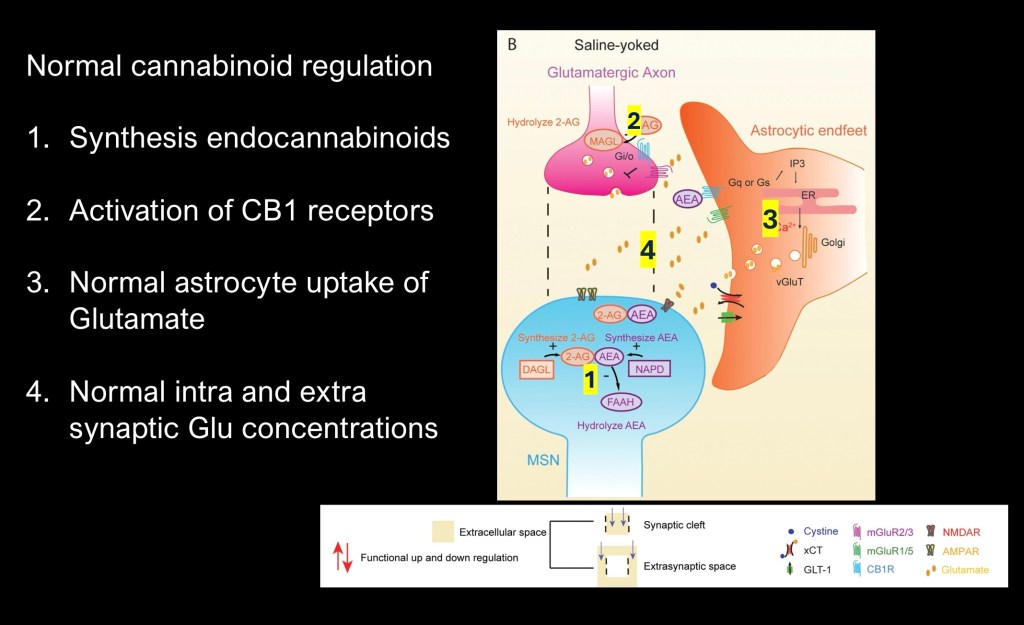
Steps involved in normal EC regulation
1. Synthesis of 2-AG and AEA.
2. 2-AG acts to inhibit Glutamate synthesis acting as a negative feedback agent.
3. AEA counteracts the glutamate-cysteine exchanger and promotes glutathione conversion of intracellular glutamate.
4. Maintains correct ratio of synaptic/extrasynaptic glutamate
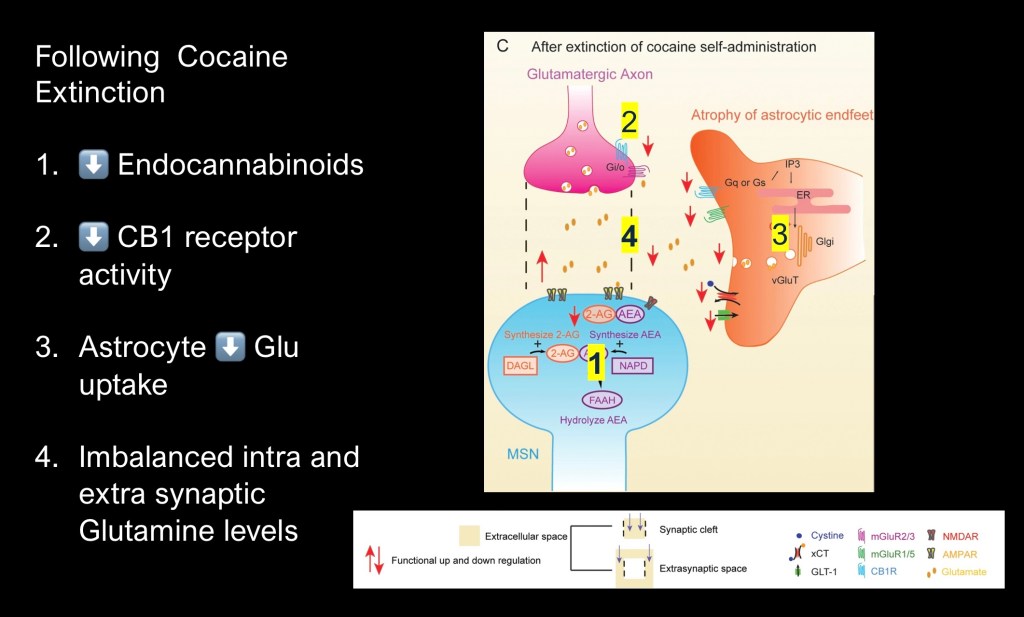
Following extinction of drug seeking behavior in cocaine dependent rodents.
1. Down regulation of endocannabinoid synthesis in post synaptic neuron
2. Decrease in down regulation of glutamate transmission by presynaptic neuron.
3. Decrease in Glutamate uptake and cycling by astrocytes
4. Glutamate imbalance promotes relapse behavior.
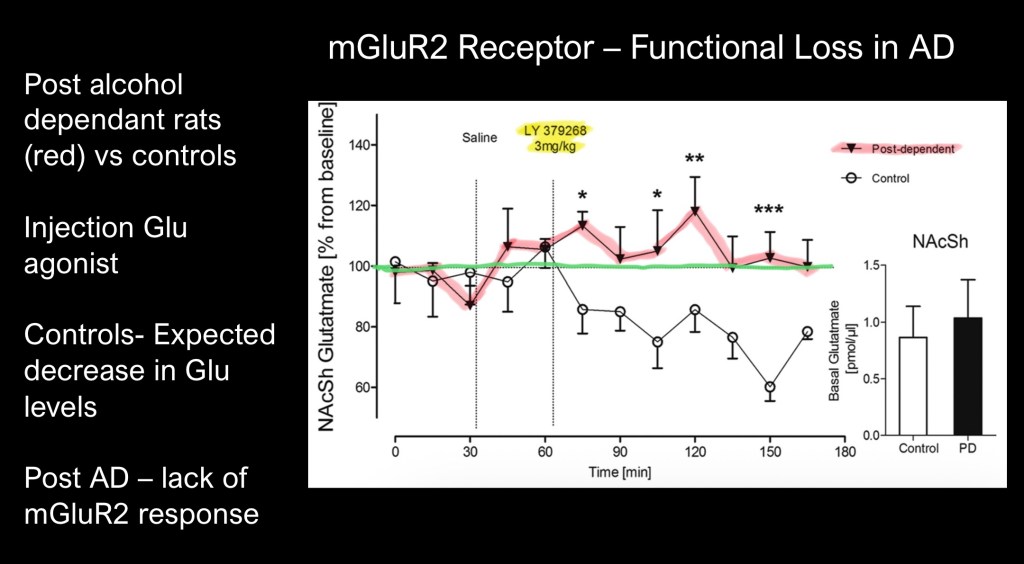
In this experiment Glutamate levels in the nucleus accumbens were measured in post alcohol dependent rats compared with a control population. The glutamate receptor against LY379268 was administered by peritoneal injection. Glutamate levels by implanted cannula were measured at baseline and post injection.
In alcohol naïve rats there is expected drop in extra cellular glutamate due to mGluR2 inhibition. Glutamate levels in alcohol dependent rats remain at baseline indicating lack of functional inhibitory control.
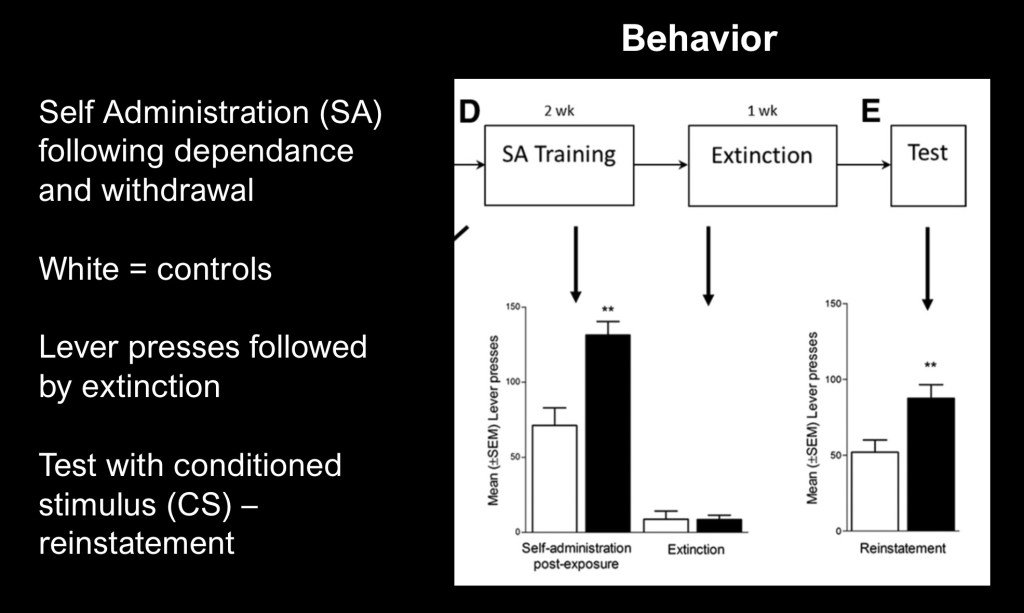
The study correlated voluntary alcohol administration corresponding to the neurobiological changes. The test group underwent alcohol dependance (AD) followed by forced abstinence and then alcohol rexposure. Columns on the left reflect mean lever presses in a two week fixed alcohol reward schedule. Black bands represent reinstatement behavior in AD rats.
In the next column both groups underwent one week extinction training. Column E represents cue induced (signal light) reinstatement without alcohol in the delivered solution. AD rats demonstrated more lever presses than controls indicating heightened cue response.
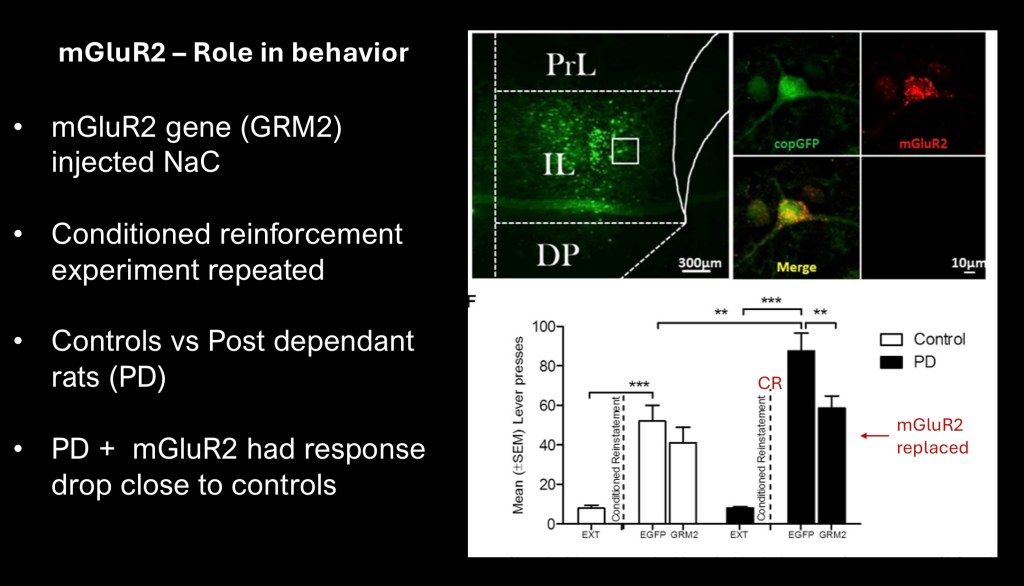
In this part of the study the investigators used a viral transgenic vector to narrow in on the specific role of mGluR2 in conditioned reinstatement of alcohol seeking in AD animals.
Using a viral vector a green fluorescent general protein marker (copGOP) was used to demonstrate successful integration of the viral DNA into host cells. Red fluorescence indicates mGluR2 gene (expressed GRM2) inserted into medial frontal cortex neurons in AD rats.
Graphs reflect lever presses with the conditioned stimulus following extinction. As expected AD rats deficient in mGluR2 show decreased inhibition. Those receiving a “rescue” DNA insertion made fewer presses indicating successful partial restoration of inhibition directly attributed to replaced mGluR2.
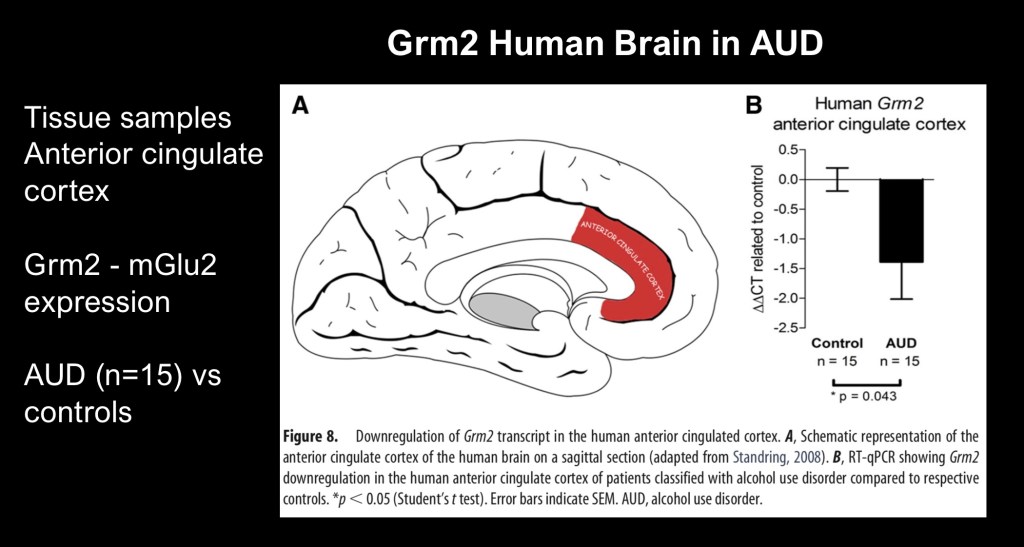
To show correlation in human brain tissue the authors analyzed levels of the precursor gene Grm2 in the anterior cingulate cortex in cadaveric brain tissue from AD and control subjects. Marked decrease in expression was present in alcohol dependent subjects.
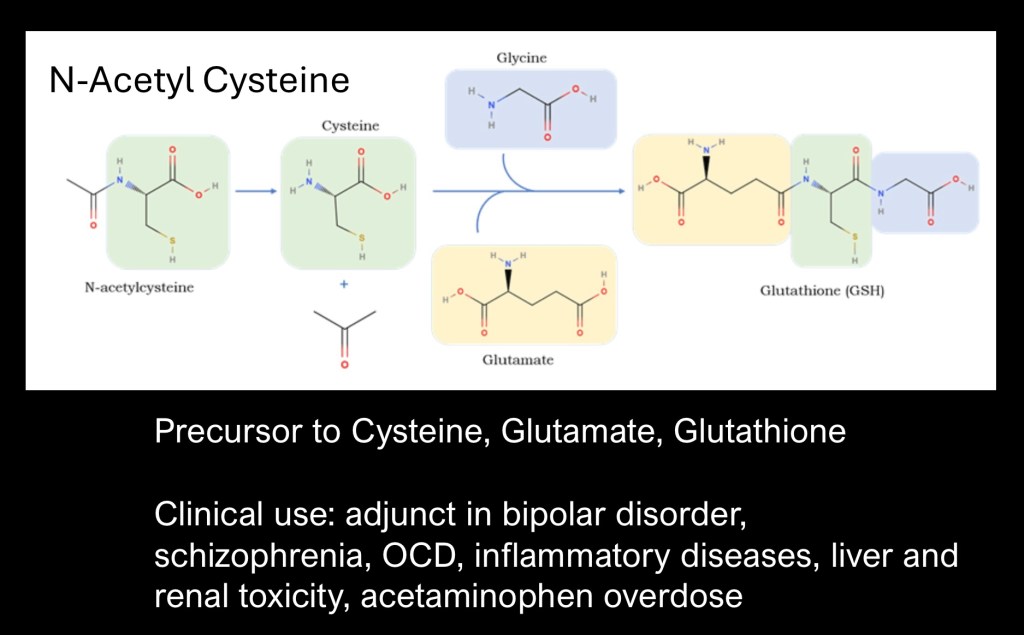
N-Acetylcysteine (NAC) is a precursor of cysteine, glutamate, and glutathione. It crosses the blood brain barrier. NAC has been used as an adjunct to treatment of psychiatric disorders such as OCD, inflammatory diseases, liver and renal toxicity, and prevention of iodinated contrast nephropathy.
It has been explored as an agent to help restore glutamate balance in substance use disorders.
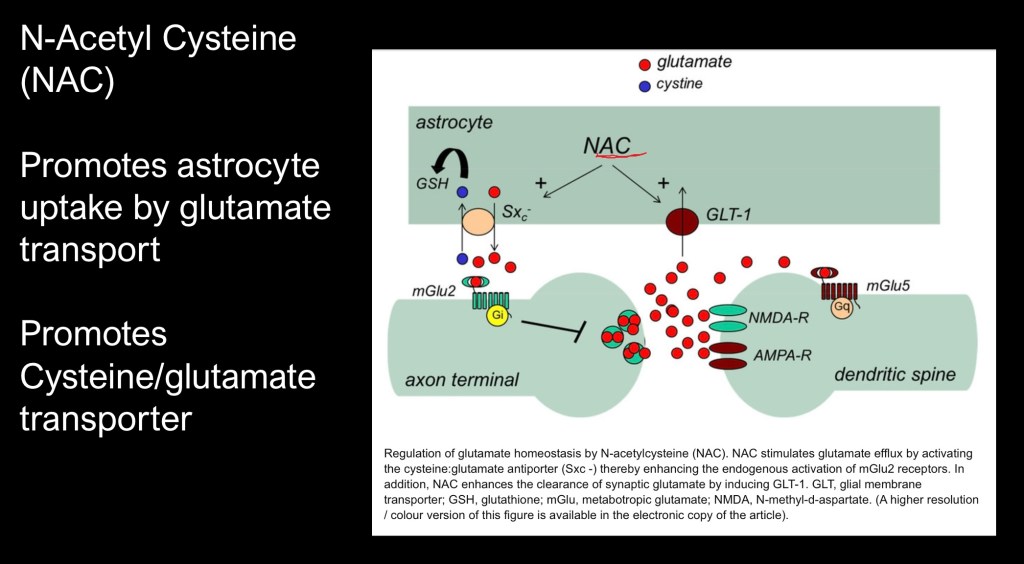
One of the mechanisms involved in Glutamate homeostasis is the astrocytic cysteine – glutamate exchanger. This surface protein transports cysteine into the cell and transports glutamate out into the extracellular space. Glutamate can then activate mGluR2 resulting in inhibitory control which has been shown to decrease drug seeking behavior in animal models.
NAC also promotes glutamate transporter activity lowering synaptic glutamate.
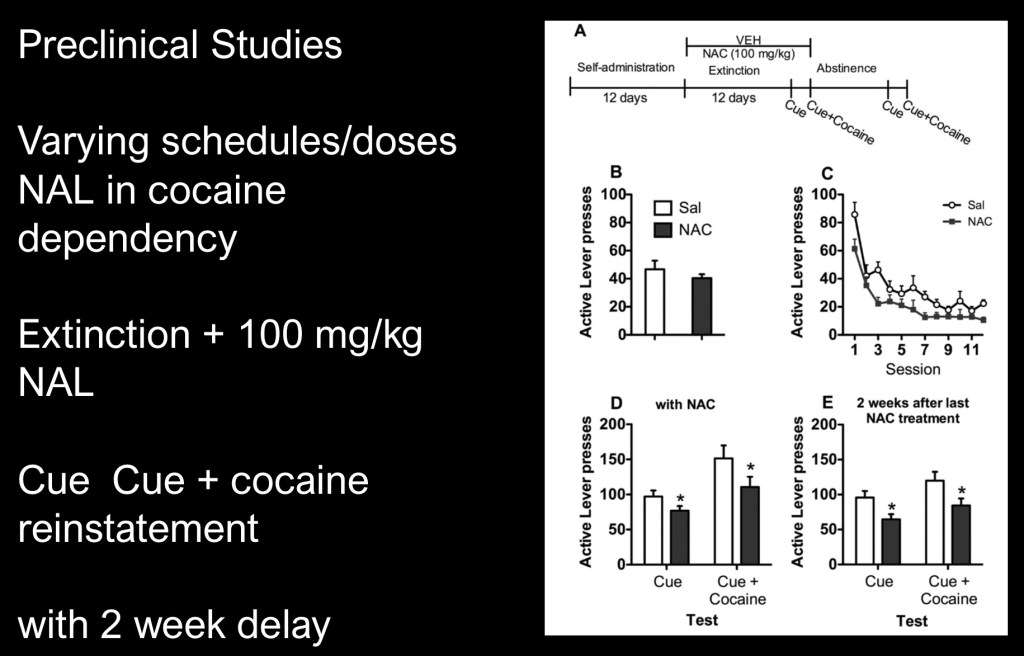
This was a multipart study in rats using a number of NAC dosing schedules in cocaine relapse and reinstatement models under varying conditions.
The top bar depicts the schedule of operant administration, extinction by unrewarded lever presses, and paired or unpaired cues. In this experiment 100mg/kg NAC was administered during extinction
There were fewer lever presses in the NAC group (black bars) indicating a positive therapeutic response.
Significantly this pattern persisted after a two week abstinence during which no NAC was given. This suggests a long term therapeutic effect.
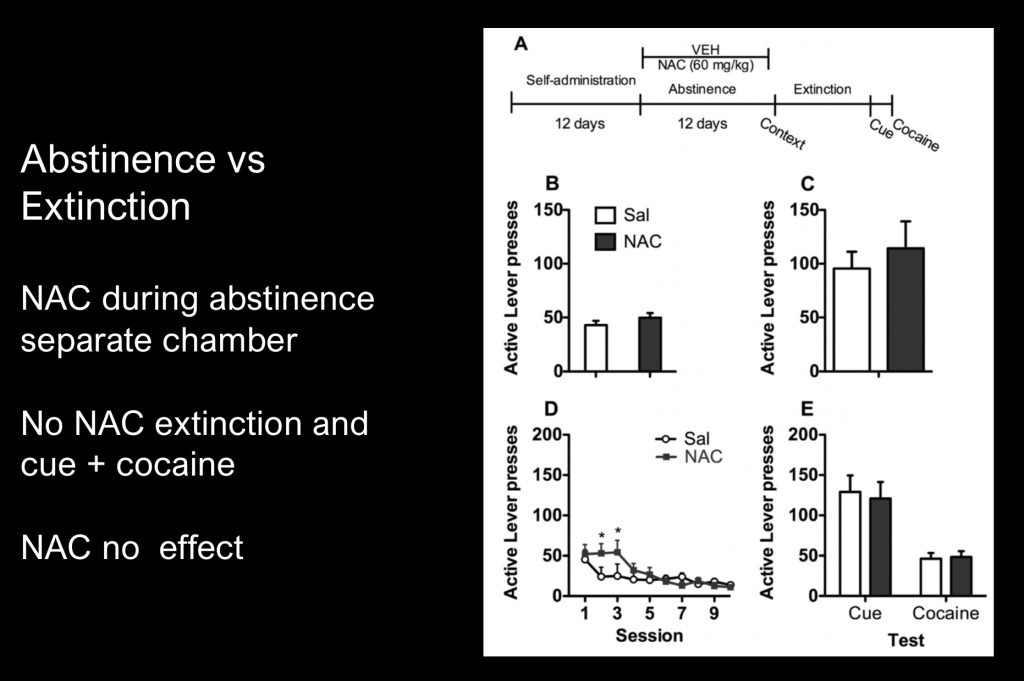
In this study abstinence means placing the animal in a cocaine free environment after a dependent state has been reached where the animals had free daily access. Self administration following abstinence is termed relapse.
When the animal is kept in the chamber where dependance developed but without drug reward when the lever is pressed the term extinction is used. Subsequent drug seeking behavior accompanied by a cue is termed reinstatement.
Relapse and reinstatement then measure different things in animal models. The experiment was designed to test these differences in N-Acetylcysteine (NAC) administration.
This phase detected no difference between test and control groups when NAC was only given during abstinence. The previous example did detect decreased appetitive behavior when NAC was given during extinction.
These findings strongly suggest that restoring glutamate homeostasis affects context driven and active operant learning and not simple abstinence.
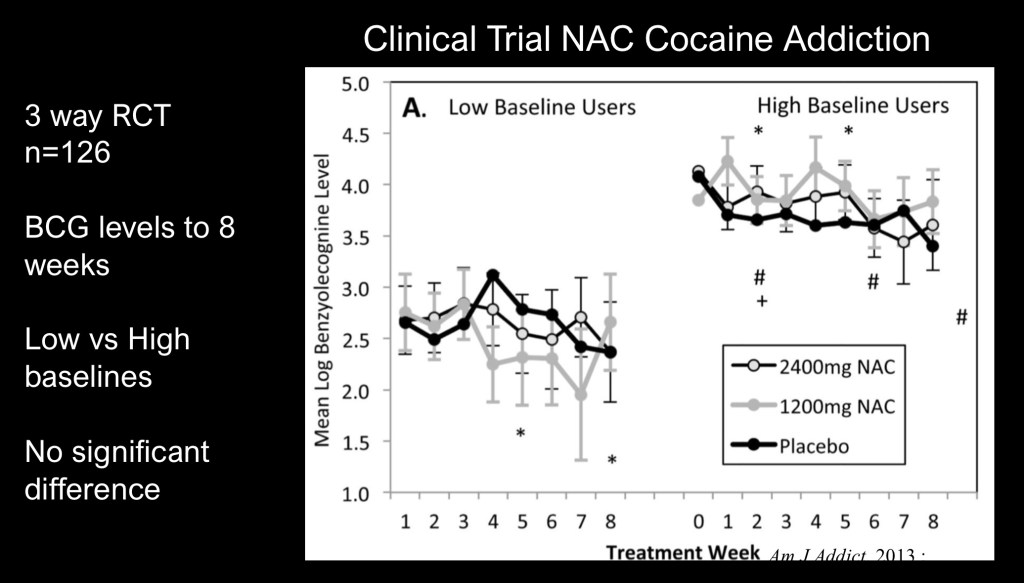
A three way naturalistic human clinical trial of low and high dose NAC in treatment of cocaine use disorder. Subjects (n=126) were grouped into low and high baseline use based on measurement of the metabolite benzolycogonine in urine.
Eight week treatment failed to show significant benefit over controls at low or high doses. Other studies have indicated overall decreased amount of intake without decreased frequency of use.
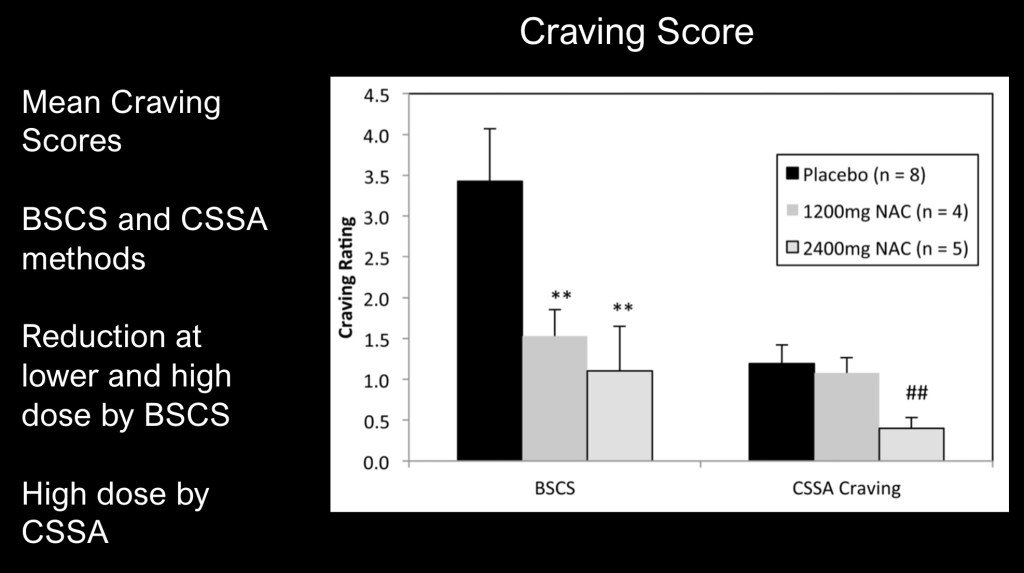
Decreased craving scores on two subjective rating scales demonstrating decreased dose dependant craving. NAC is sometimes used off label in cocaine use disorder as an adjunct to psychosocial intervention.

Glutamate is the most abundant excitatory neurotransmitter in the brain and is present in around 90% of neural synapses. Glutamate levels within and around neural synapses are tightly controlled and small deviations may have profound consequences.
The tripartite synapse is the functional unit consisting of pre and post synaptic neurons and surrounding astrocytes. An array of receptors and transporters regulate glutamate levels. Research has focused on the mGluR2 receptors which function as feedback negative regulators of glutamate transmission. These receptors have been shown to be under expressed in substance use disorders resulting in long-term imbalance contributing to relapse.

The Glutamate Homeostasis theory of addiction focuses on the Cortical-striatal pathway from the infra limbic cortex to the Nucleus Accumbens and dorsal stratum as a major component contributing to relapse in SUD.
N-acetyl cysteine has shown promise as a therapeutic agent helping to restore glutamate imbalance in animal models. In human trials NAC has demonstrated mixed results. There is potential for development of more specific targeted agents in the future as pharmacogenetics and computational modeling with advances in AI and quantum computing come online.

For information and educational purposes only. Data and images obtained from sources freely available on the World Wide Web. This post should not be considered medical or professional advice
References
Psychological and neural mechanisms of relapse
Jane Stewart*
Center for Studies in Behavioral Neurobiology/Groupe de Recherche en Neurobiologie Comportementale,
Department of Psychology, Concordia University, 7141 Sherbrooke Street West,
Montreal, Quebec, Canada H4B 1R6
Phil. Trans. R. Soc. B (2008) 363, 3147–3158
doi:10.1098/rstb.2008.0084 Published online 18 July 2008
……………………………………………………………………………..
The reinstatement model of drug relapse: recent neurobiological findings, emerging research topics, and translational research
Jennifer M Bossert 1, Nathan J Marchant 1, Donna J Calu 1, Yavin Shaham
Published in final edited form as: Psycho pharmacology (Berl). 2013 May 18;229(3):453–476. doi: 10.1007/s00213- 013-3120-y
https://pmc.ncbi.nlm.nih.gov/articles/PMC3770775/
Group II metabotropic glutamate receptors (mGlu2/3) in drug addiction
Khaled Moussawi 1, Peter W Kalivas 1
Eur J Pharmacol. 2010 Apr 2;639(0):115–122. doi: 10.1016/j.ejphar.2010.01.030
https://pmc.ncbi.nlm.nih.gov/articles/PMC4351804/
Kalivas, P. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci10, 561–572 (2009). https://doi.org/10.1038/nrn2515
Published01 July 2009 Issue DateAugust 2009 DOIhttps://doi.org/10.1038/nrn2515
https://www.nature.com/articles/nrn2515
mGlu2 mechanism-based interventions to treat alcohol relapse
Vengeliene V and Spanagel R (2022), mGlu2 mechanism-based interventions
to treat alcohol relapse.
Front. Pharmacol. 13:985954.
doi: 10.3389/fphar.2022.985954
https://www.frontiersin.org/journals/pharmacology/articles/10.3389/fphar.2022.985954/pdf
……………..”………………………………………………
Disrupted Ventromedial Prefrontal Function, Alcohol Craving,
and Subsequent Relapse Risk
Dongju Seo, PhD, Cheryl M. Lacadie, BA, Keri Tuit, PsyD, Kwang-Ik Hong, MS, R. Todd Constable, PhD, and Rajita Sinha, PhD
Departments of Psychiatry (Drs Seo, Tuit, and Sinha, and Mr Hong) and Diagnostic Radiology . Ms Lacadie and Dr Constable), Yale University School of Medicine, New Haven, Connecticut
JAMA Psychiatry . 2013 July ; 70(7): 727–739. doi:10.1001/jamapsychiatry.2013.762.
…………………………………………………………………………………………
McHugh, M. J., Demers, C. H., Braud, J., Briggs, R., Adinoff, B., & Stein, E. A. (2013). Striatal-insula circuits in cocaine addiction: implications for impulsivity and relapse risk. The American Journal of Drug and Alcohol Abuse, 39(6), 424–432. https://doi.org/10.3109/00952990.2013.847446
https://www.tandfonline.com/doi/full/10.3109/00952990.2013.847446
…………………………“……………………”?……….
The glutamate homeostasis
hypothesis of addiction
173 Ashley Avenue, BSB 410, Department of Neurosciences, Medical University of South Carolina, Charleston, South Carolina 29425, USA. e‑mail: kalivasp@musc.edu doi:10.1038/nrn2515 Published online 1 July 2009 Peter W. Kalivas
NATuRe RevIews | NeuroscieNce volume 10 | AugusT 2009 | 561
Dysfunctional approach behavior triggered by alcohol-unrelated Pavlovian cues predicts long-term relapse in alcohol dependence
Christian Sommer, Julian Birkenstock, Maria Garbusow, Elisabeth Obst, Daniel J. Schad, Nadine Bernhardt,
Journal of Neuroscience 29 April 2009, 29 (17) 5389-5401; https://doi.org/10.1523/JNEUROSCI.5129-08.2009
https://www.jneurosci.org/content/29/17/5389
Astrocytic Glutamatergic Transmission and Its Implications in Neurodegenerative Disorders
by Sairaj Satarker, Sree Lalitha Bojja
Cells 2022, 11(7), 1139; https://doi.org/10.3390/cells11071139
https://www.mdpi.com/2073-4409/11/7/1139
Astrocytes Maintain Glutamate Homeostasis in the CNS by Controlling the Balance between Glutamate Uptake and Release
by Shaimaa Mahmoud, Marjan Gharagozloo, Camille Simard and Denis Gris *
Program of Immunology, Department of Pharmacology-Physiology, Faculty of Medicine and Health Sciences, University of Sherbrooke, Sherbrooke, QC J1H 5N4, Canada
Cells 2019, 8(2), 184; https://doi.org/10.3390/cells8020184
https://www.mdpi.com/2073-4409/8/2/184
Alterations in the morphology of dendrites and dendritic
spines in the nucleus accumbens and prefrontal cortex
following repeated treatment with amphetamine or
cocaine
Terry E. Robinson and Bryan Kolb1
Department of Psychology and Neuroscience Program, The University of Michigan, 525 E. University, Ann Arbor,
MI 48109, USA
1Department of Psychology, University of Lethbridge, Lethbridge, Alberta, Canada T1K 3M4
European Journal of Neuroscience, Vol. 11, pp. 1598–1604, 1999
“…………………………………………………………………………………
“……………………………………………………………………………………
Long-term potentiation and long-term depression: a clinical perspective
Timothy VP Bliss I, Sam F Cooke II
Clinics (Sao Paulo). 2011 Jun;66(Suppl 1):3–17. doi: 10.1590/S1807-59322011001300002
https://pmc.ncbi.nlm.nih.gov/articles/PMC3118435/
……………………………………………………………………………..
Corticostriatal circuitry
Dialogues Clin Neurosci. 2016 Mar;18(1):7–21. doi: 10.31887/DCNS.2016.18.1/shaber
https://pmc.ncbi.nlm.nih.gov/articles/PMC4826773/
The Nucleus Accumbens: Mechanisms of Addiction
across Drug Classes Reflect the Importance of
Glutamate Homeostasis
M. D. Scofield, J. A. Heinsbroek, C. D. Gipson, Y. M. Kupchik
1521-0081/68/3/816–871$25.00 http://dx.doi.org/10.1124/pr.116.012484
Pharmacol Rev 68:816–871, July 2016
Department of Neuroscience, Medical University of South Carolina, Charleston, South Carolina (M.D.S., J.A.H., S.S., D.R.-W., P.W.K.);
Department of Psychology, Arizona State University, Tempe, Arizona (C.D.G.); Department of Neuroscience, Hebrew University, Jerusalem,
Israel (Y.M.K.); and Department of Pharmacology and Systems Therapeutics, Icahn School of Medicine at Mount Sinai, New York,
MyAstrocytic Dysfunction and Addiction: Consequences of
Impaired Glutamate Homeostasis
Michael D. Scofield1 and Peter W. Kalivas1
1Medical University of South Carolina, Charleston, SC, USA, Department of Neurosciences
Neuroscientist . 2014 December ; 20(6): 610–622. doi:10.1177/1073858413520347.
……………………………………………………………
Rescue of Infralimbic mGluR2 Deficit Restores Control Over Drug-Seeking Behavior in Alcohol Dependence
Marcus W. Meinhardt, Anita C. Hansson, Stephanie Perreau-Lenz, Christina Bauder-Wenz, Oliver Stählin, Markus Heilig, Clive Harper, Karla U. Drescher, Rainer Spanagel and Wolfgang H. Sommer
Journal of Neuroscience 13 February 2013, 33 (7) 2794-2806; https://doi.org/10.1523/JNEUROSCI.4062-12.2013
https://www.jneurosci.org/content/33/7/2794/tab-rc
………………………………………………………………….
Molecular Basis for Modulation of Metabotropic Glutamate Receptors and Their Drug Actions by Extracellular Ca2+
by Juan Zou †, Jason Y. Jiang † and Jenny J. Yang *
Department of Chemistry, Center for Diagnostics and Therapeutics, Georgia State University, Atlanta, GA 30303, USA
Int. J. Mol. Sci. 2017, 18(3), 672; https://doi.org/10.3390/ijms18030672
https://www.mdpi.com/1422-0067/18/3/672
……………………………………………………………………..
Regulation of glutamate homeostasis in the nucleus accumbens by astrocytic CB1 receptors and its role in cocaine-motivated behaviors
Lan-Yuan Zhang ,Andrew Y. Kim , Joseph F. Cheer
Addiction Neuroscience Volume 3, September 2022, 100022
Ohttps://www.sciencedirect.com/science/article/pii/S2772392522000177
A Double-Blind Placebo-Controlled Trial of N-Acetylcysteine in
the Treatment of Cocaine Dependence
Steven D. LaRowe, Ph.D., Peter W. Kalivas, Ph.D.
Am J Addict. 2013 ; 22(5): 443–452. doi:10.1111/j.1521-0391.2013.12034.x.
Chronic N-Acetylcysteine during Abstinence or Extinction after Cocaine Self-Administration Produces Enduring Reductions in Drug Seeking
Carmela M. Reichel, Khaled Moussawi, Phong H. Do,
THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS Copyright © 2011 by The American Society for Pharmacology and Experimental Therapeutics JPET 337:487–493, 201
…………………………………………………………

Leave a comment