
This post focuses on the neurobiology of what has been termed the “dark side” of addiction. This encompasses dysphoria, stress and anxiety as it pertains to substance addictions. While drug use is initially driven by positive affect and hedonic reward there are physiologic shifts which occur with chronic use. Over time negative reward comes to dominate behavior as homeostatic set points can no longer be maintained. Resulting neuroadaptive change then oscillates around newer more negative set points, a process known as allostasis.

In physics stress can be defined as force per unit area. In biology stress is difficult to precisely define. It can be thought of as internal or external factors disrupting homeostatic mechanisms. Adaptation to stress is a normal function for all organisms. However excessive stress or disruption of compensatory regulating factors can be highly problematic.
We have multilevel systems for processing and appropriately dealing with stressors in the long and short term. Addictive drugs may initially be used as self medication to control stress and anxiety. As addictive use progresses drug use itself becomes the source of maladaptive responses and additional negative load.
This post reviews the major stress related neuro physiology involving the hypothalamic – pituitary – adrenal axis and extended amygdala along with the role of glucocorticoids and opiate systems with attention to adaptive changes occurring in addiction and other pathologies.

The brain disease model of addiction introduced by Nora Volkow and George Koob at the NIH is represented as a cycle composed of three components. Initial use is primarily driven by positive reward. Over time negative affect, dysphoria, and excess stress are the principal drivers of continued drug use. Preoccupation with drug related cues is progressive and involves cognitive dysfunction and narrowed pursuit of other life priorities such as work, social, and family life.

Opponent – Process Theory is graphically represented above. Acute effects are toward the left. Chronic effects are in the right column. The top (green) line represents drug self administration. The middle line reflects opposing (red) positive reward A – process and the (blue) dysphoric B -process.
Summation of A and B (black) yields a net deficit in the chronic state. The new “normal” is termed allostasis.
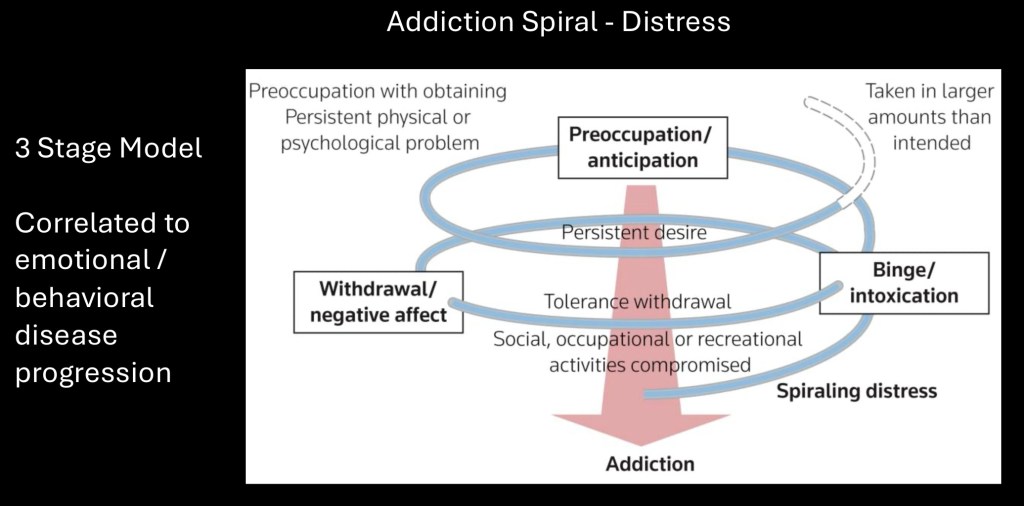
The addiction cycle may be represented as progressive emotional and behavioral maladaptive changes with loss of control, physical, and functional consequences. Increasing severity of distress promotes increased drug use in an attempt to temporarily relieve negative symptoms. At every point profound disruption of balanced regulatory mechanisms, many of them long term, occurs in a downward trajectory.
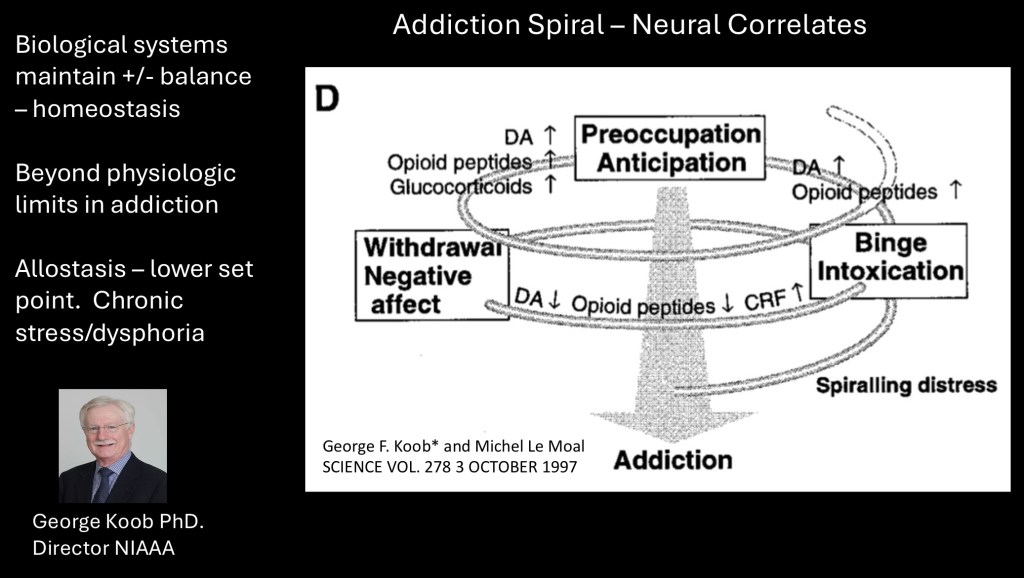
Changes in neurotransmitter levels correlate to emotional and behavioral experience. Dopamine and endorphin levels are initially elevated with each dose corresponding to positive reward. Further down these two neurotransmitters decline and output of Corticotropin Releasing Factor (CRF) from the hypothalamus, the initial step in stress reactions, increases.
George Koob PhD is currently director of the NIAAA, formerly with Scripps Research institute. He has been the principal investigator of the negative reinforcement aspect of the addiction cycle. Much of this post is taken from some of the many articles and research studies he has published.
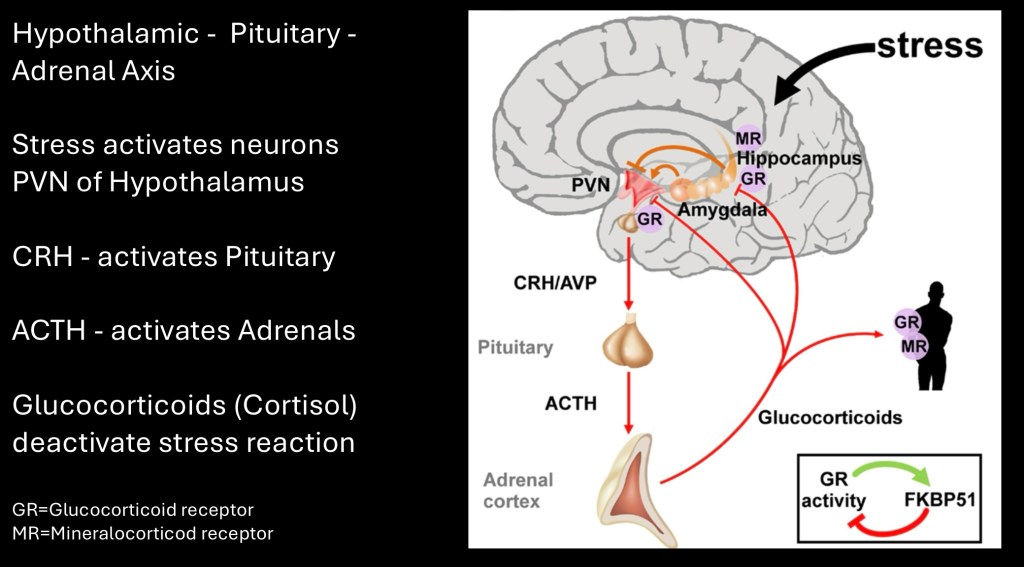
Acute and chronic stress reaction is not a single process. It involves multilevel interactive pathways involving the central and peripheral nervous systems.
The hypothalamic-pituitary-adrenal axis (HPA) originates in the hypothalamic paraventricular nucleus (PVN) at the base of the brain. When a stress inducing signal activates the PVN the neuro peptide hormone Corticotropin Releasing Hormone (also known as CRF) is released. The process may be initiated by physical or emotional stress, perceived threats, infection, pain, and chronic use of addictive drugs such as alcohol (SUD).
CRF travels to the anterior pituitary gland where the hormone Adrenocortical Tropic Hormone (ACTH) is released into the bloodstream.

The adrenal glands are triangular organs located just above the kidneys. The functional anatomy consists of an outer cortex which can be subdivided into three zones and an inner medulla.
The cortex produces the mineralocorticoid Aldosterone important in maintaining mineral balance. It also produces glucocorticoids, in humans chiefly the stress hormone Cortisol. The medulla produces the catecholamine neurotransmitters Epinephrine (Adrenalin), and closely related Norepinephrine.

The autonomic nervous system consists of two divisions. The sympathetic system is responsible for the “flight or fight” response. It originates primarily from paraspinal nerve roots. The sympathetic system is driven by the catecholamine neurotransmitter epinephrine and prepares the body for immediate action to real or perceived threats. Effects extend to the cardiovascular system, lungs, digestive organs, sex organs, and other structures. It raises heart rate and blood pressure, shunts blood to central circulation, and slows digestive activity.
The parasympathetic system is mediated by acetylcholine and is responsible for opposing “rest and digest” activity. It lowers heart rate and blood pressure and promotes bowel motility. It originates from the Vagus and pelvic nerves.
Additional information on the role of the autonomic nervous system here

Basic diagram of the HPA feedback loop. The glucocorticoids are a hormone group of which Cortisol is the primary human stress related hormone. It acts on glucocorticoid receptors located in the hypothalamus, anterior pituitary, and various sites throughout the brain.
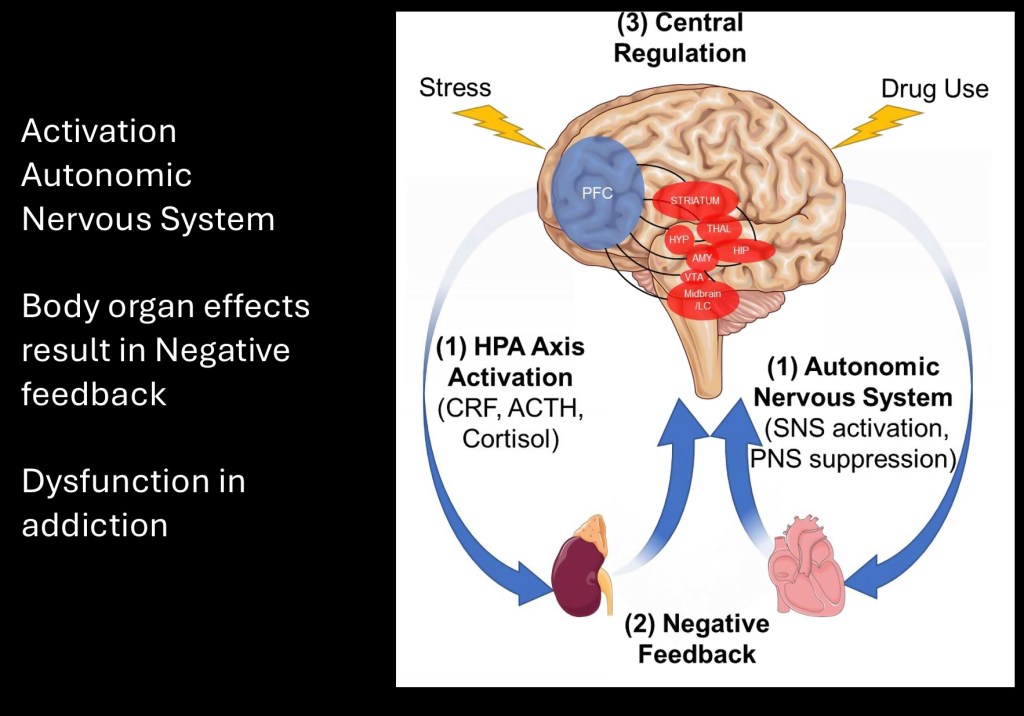
In addition to cortisol secreted by the adrenal glands sensory information from the cardiovascular system and visceral organs provide feedback to central brain structures. Chronic SUD results in stress overload due to maladaptive allostatic compensatory mechanisms.
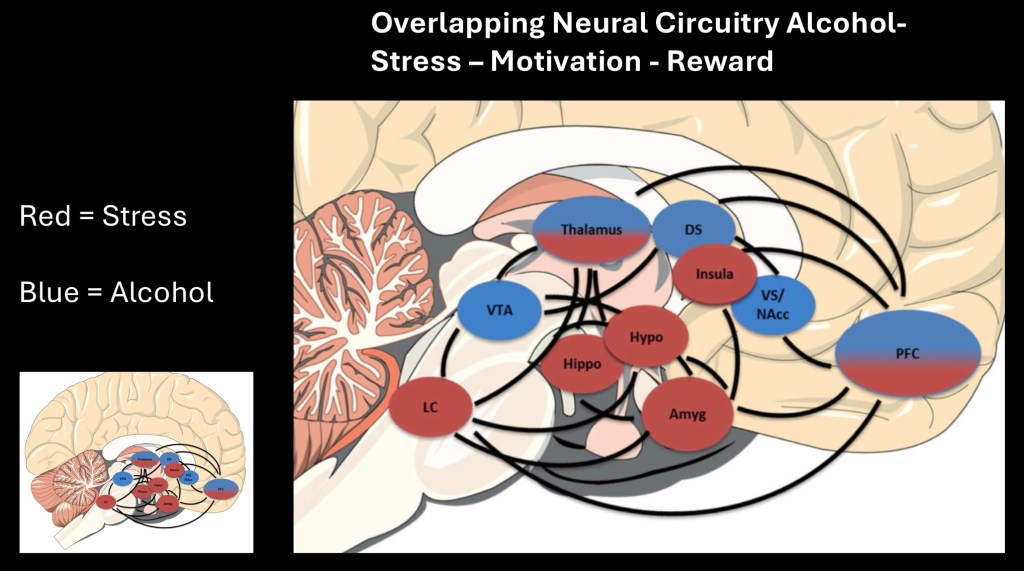
This represents brain structures affected in Alcohol use disorder and chronic stress and functional connecting pathways. Blue indicates structures primarily influenced by alcohol. Red primarily affected by stress with or without AUD. Note overlap in the Thalamus and prefrontal cortex. Stress reactions are complex and include higher level motivation, reward, avoidance and cognitive reasoning.
j

In a normal stress response cortisol effectively activates glucocorticoid receptors. Serum cortisol acts to regulate blood pressure, releases glucose stores from the liver, and lowers inflammation. Chronic stress results in high cortisol levels increasing inflammation and disturbing normal brain function. Continued chronic stress reaction causes cortisol resistance in the hypothalamus and adrenal glands resulting in increased inflammation and neural damage.

Neural responses to stress activates several systems both subconscious and cognitive. The salience and executive networks are activated along with the limbic and reward systems.
– The external or internal stressor activates brain centers responsible for alarm and evaluation processing.
– Salience network integrates information and activates the HPA and emotional response.
– The limbic system and reward pathways are activated. The stimulus is appraised through long and short term memory to evoke an appropriate level of activity and control.
– Integration through the executive network and adaptive changes are generated

FKBP5 is a protein modulating glucocorticoid receptor activity and a key component in overall stress reaction and HPA activation at the cellular level. When the gene coding for FKBP5 is activated it decreases the level of cortisol produced.

Normally FKBP5 blocks cortisol production by replacing it with the GR receptor in the cell. This creates a complex binding to DNA in the nucleus promoting increased expression of the FKBD5 gene and preventing HPA activation thus controlling stress reaction.
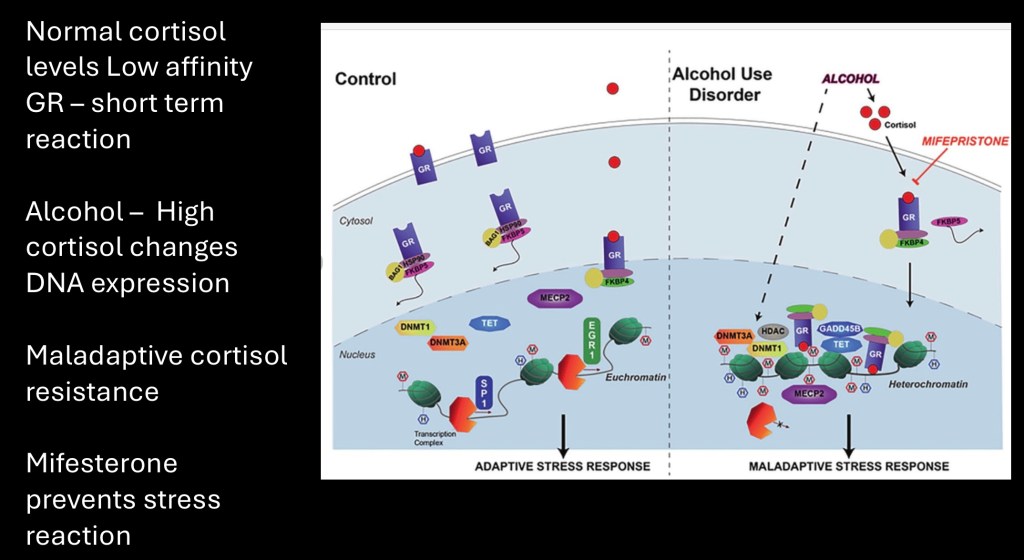
Epigenetic changes occurring in childhood trauma and chronic alcohol use interfere with this mechanism resulting in higher baseline cortisol levels. This is thought to contribute to risk of depression, PTSD, and SUD. The drug mifepristone has been shown to interfere with this mechanism decreasing drug seeking behavior in laboratory animals. It has not proven effective in human trials.
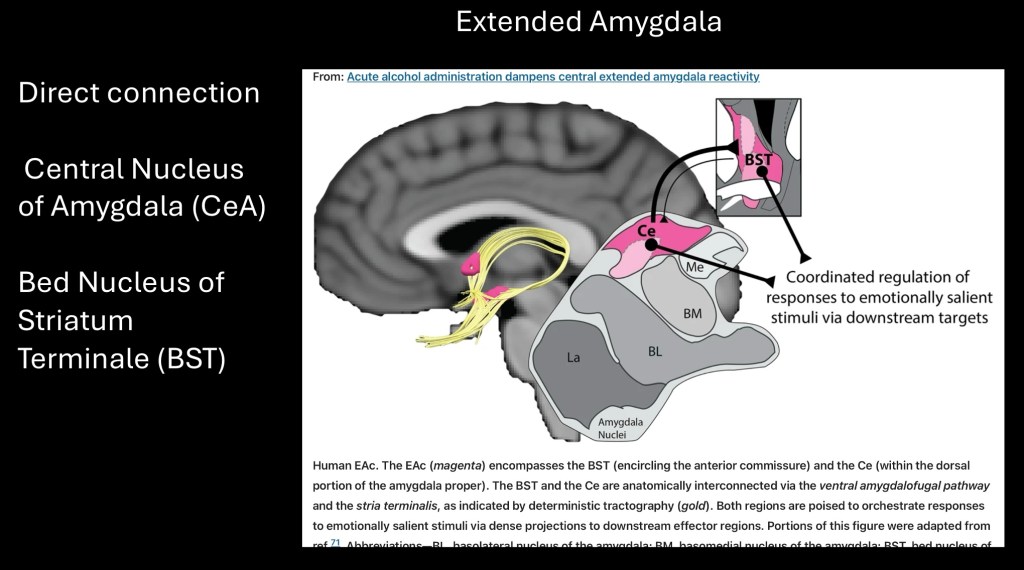
The Amydala can be subdivided into five nuclei. The extended Amygdala encompasses the central nucleus of the Amygdala and the bed nucleus of the Striatum Terminale shown in pink above. These have direct communication and together coordinate responses to emotional stimuli by communication with other brain regions.
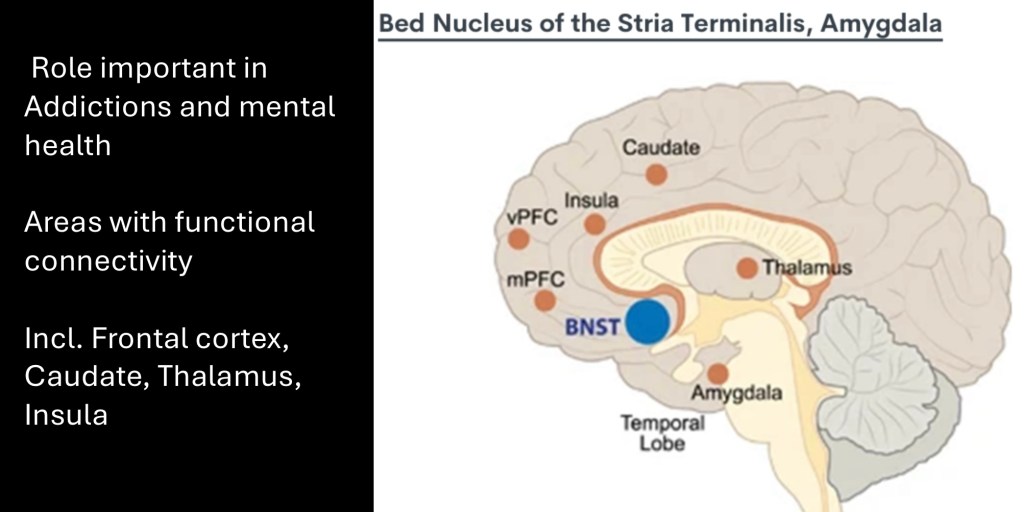
The extended Amygdala communicates with the ventral and medial prefrontal cortex, thalamus, caudate lobe and temporal insula. These areas have roles in memory retrieval, motor function, integration of sensory information, and decision making.
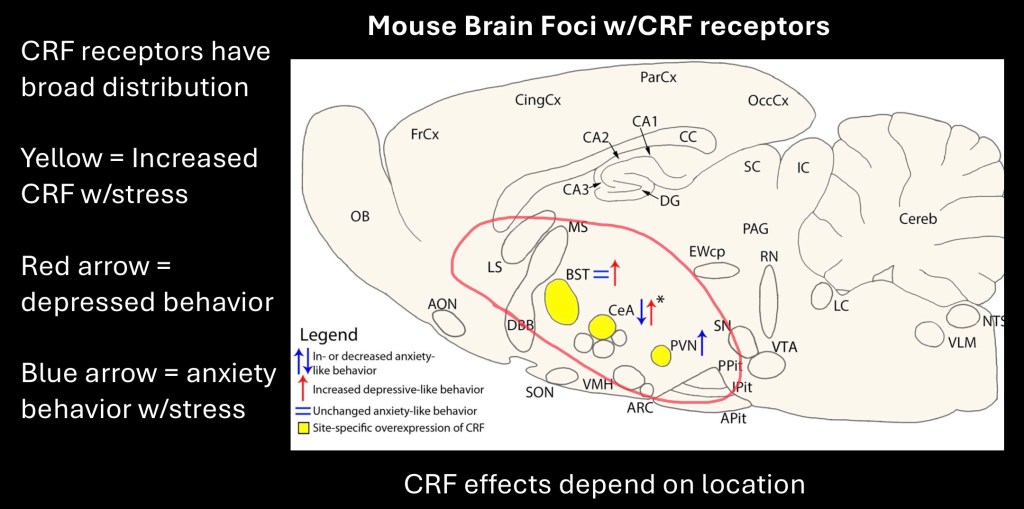
Schematic diagram of the rat brain. In addition to its role in activation of the HPA axis, Corticotropin Releasing Factor receptors are located in multiple areas of the brain including the extended Amygdala.
CRF effects have differing roles depending on location. Red arrows indicate depressive behaviors seen in preclinical studies which become increased with CRF elevation in the amygdala and BST. Blue arrows indicate anxiety behaviors which increase in the Paraventricular nucleus and decrease in the CeA.
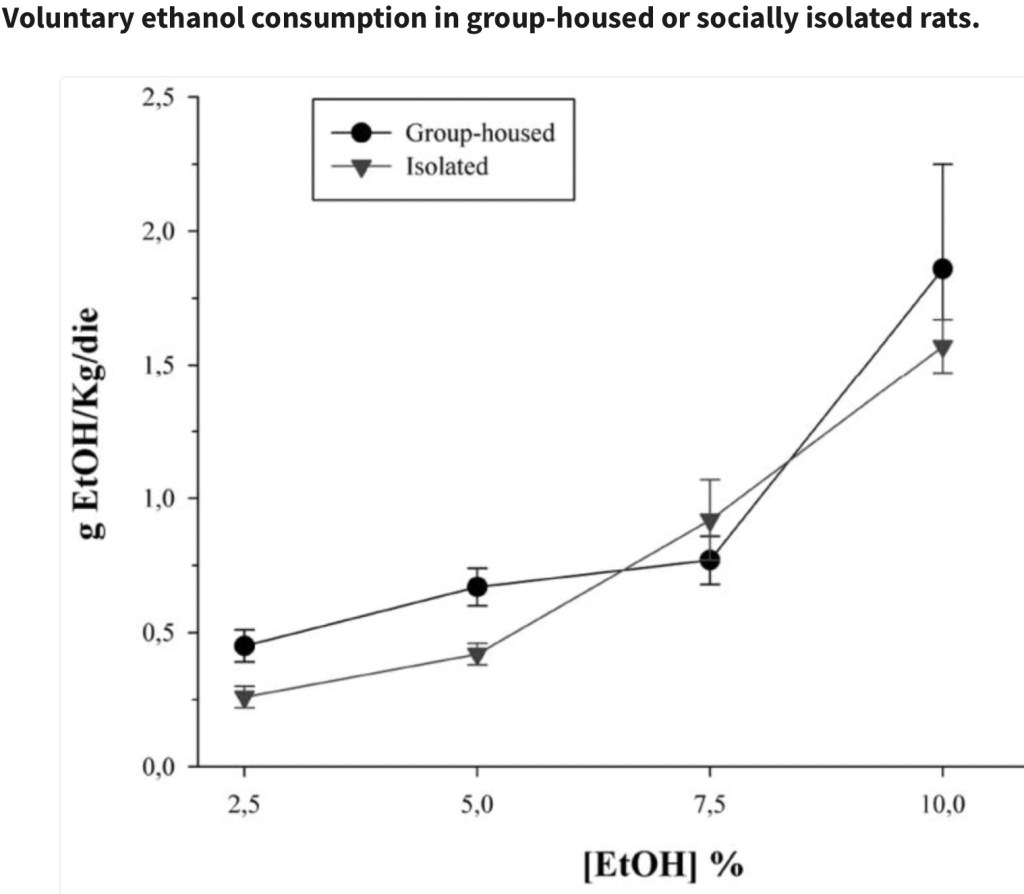
This experiment investigated the effects of voluntary ethanol consumption and social isolation on stress biomarkers and behavior in rats. The rats were separated after weaning into either isolated or group (3/cage) housing. During the experimental phase half of each group were provided either a two bottle choice of water and increasing concentrations of ethanol solution or a control group of water only. This was done for a total of 4 weeks.
The above reflects voluntary ethanol consumption comparing isolated vs group housed rats. No significant difference in overall consumption was observed between the two groups.
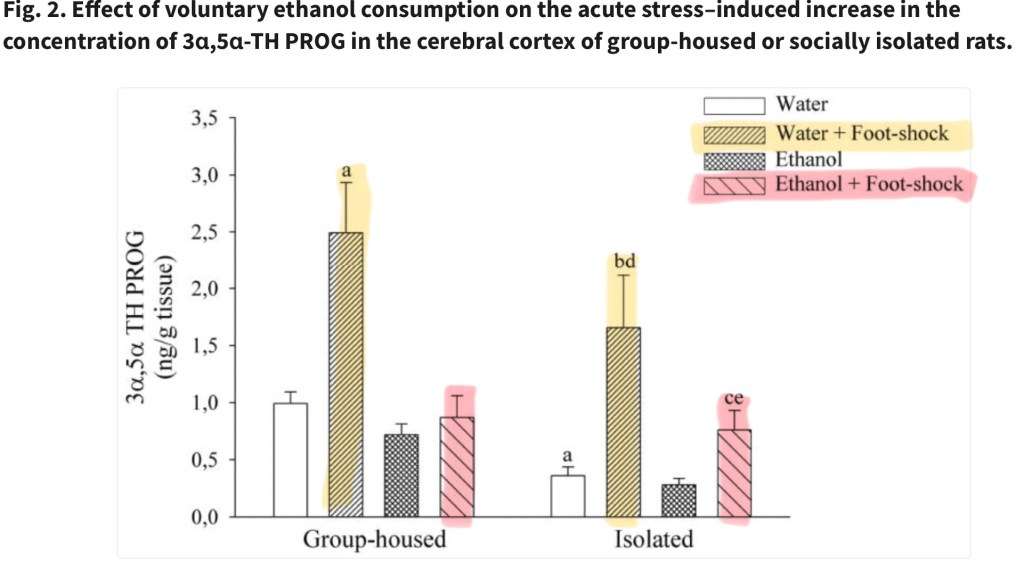
Effect of foot shock stress on levels of the glucocorticoid hormone 3 α5α-TH PROG Alcohol vs Water only in both group and isolated rats. Non stressed rats from both groups were included.
Highest levels of stress hormone were found in the water only/group-housed rats. Significantly lower levels of the hormone were found in the ethanol group. Behavioral maze test demonstrated corresponding differences in anxiety related behaviors.

In this experiment subjects were asked to identify the emotions depicted in a series of faces. This test is known as the emotion recognition test (ERT). The subjects performed this task with and without self administration of acute alcohol during acquisition of a functional MRI (fMRI). The goal was to assess the emotional sensitivity and degree of activity involving the Stress pathway.
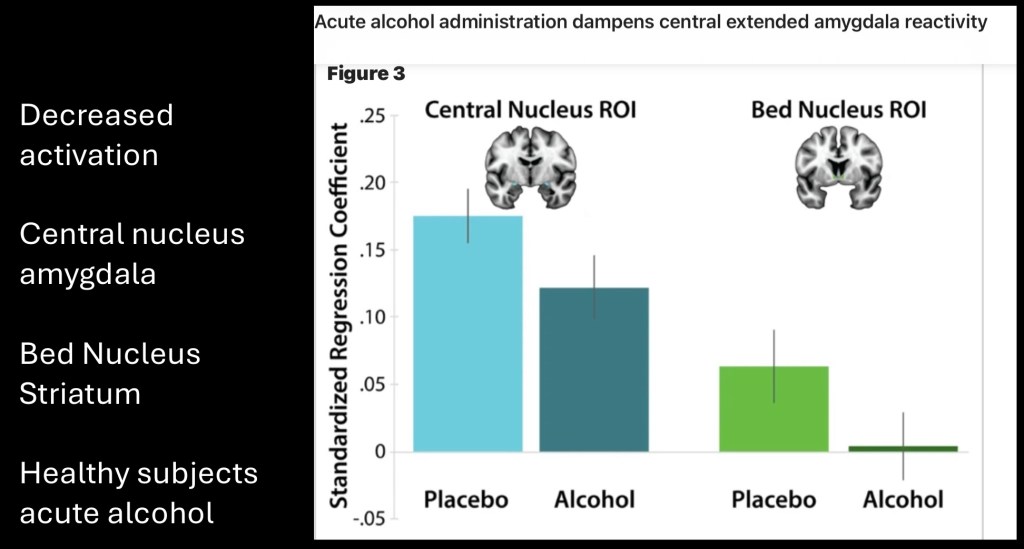
Regression coefficient reflects activity relative to ERT score at baseline and following acute alcohol administration. Significant decreases were seen in the CeA and BST post alcohol compared to control subjects. This indicates dampening of functional activation of stress and fear related arousal following a single dose of alcohol. This explains the well known reduction in stress anxiety with smaller acute doses of alcohol.
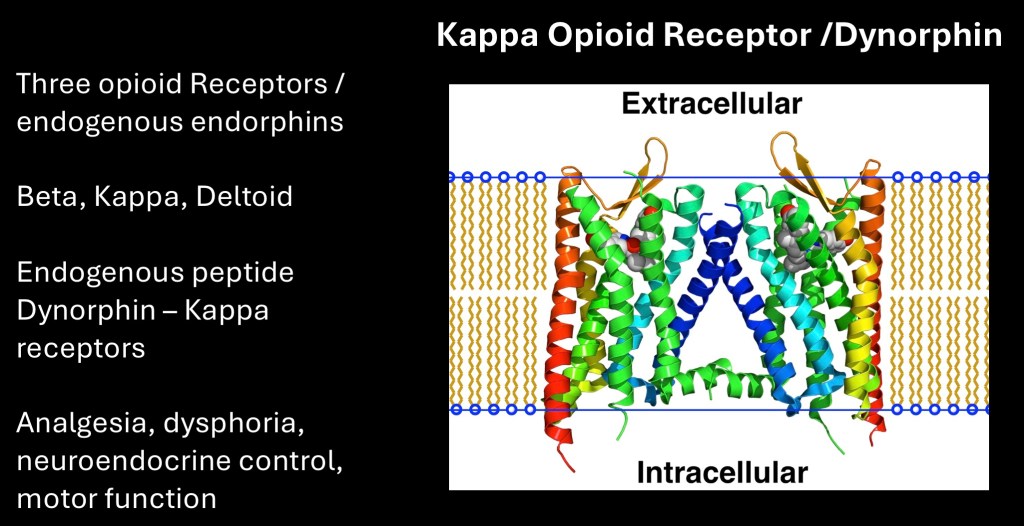
The kappa opioid receptor / Dynorphin system is another stress related pathway.
There are three types of opioid receptors, beta β, kappa κ, and delta δ. All three are involved in analgesia. The kappa receptors interact with the neuro peptide Dynorphin. When activated this results in dysphoria as opposed to the euphoric effects associated with opiate beta agonists
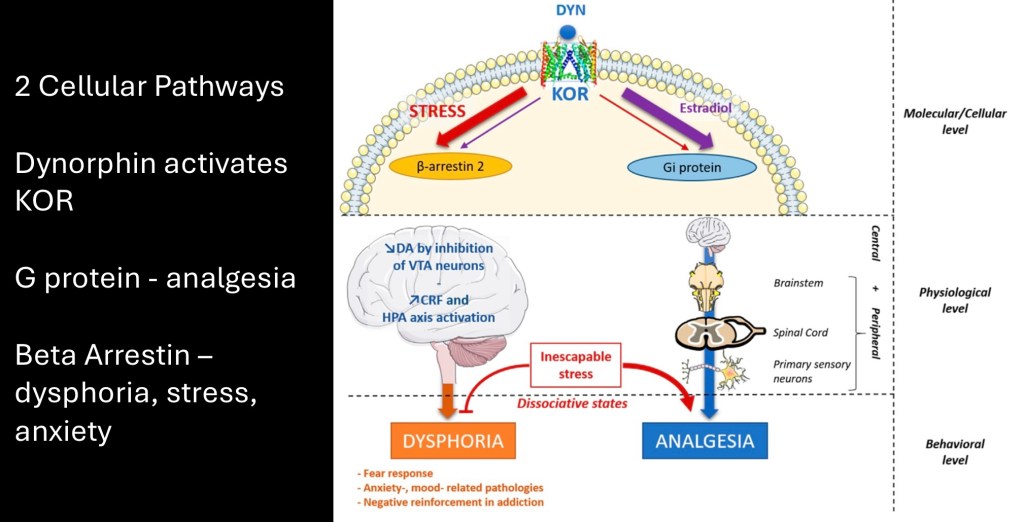
There are two cellular pathways connected to kappa receptors. The β-arrestin pathway suppresses the dopamine reward pathway and increases cortisol resulting in dysphoria. The G protein pathway extends through the spinal cord providing central analgesia.
Subjective effects result in dysphoria, anxiety, negative reinforcement and increased drug seeking behavior in an attempt to maintain homeostatic balance. In inescapable stressful situations dissociative states may arise.

Y shaped (purple) symbols depict κ opioid receptors. The diagram represents structures involved in fear and emotional responses. The amygdala seen centrally in blue drives conditioned fear response communicating with the periaquaductal gray. Also in blue is bidirectional communication with the hippocampus forming a fear and emotional contextual memory circuit.
The dopaminergic reward pathway in red, is suppressed. In later stages negative anhedonic reward drives further drug use. Communication with the prefrontal cortex results in cognitive, behavioral and subjective emotional response.
Kappa receptors and Dynorphin are involved at multiple levels in these interconnected neural networks. This provides potential targets for ongoing research and development of therapeutic agents.
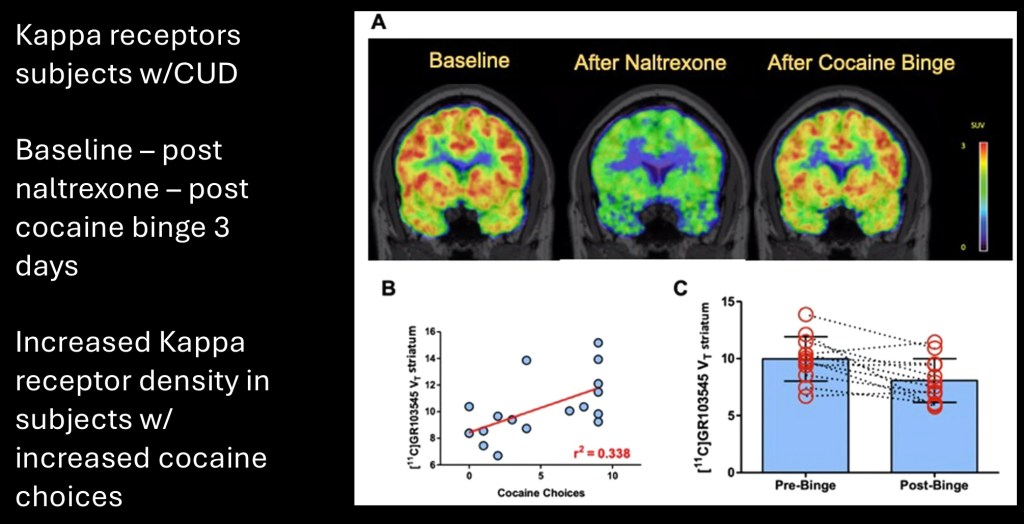
This study looked at the role of kappa opioid receptors in subjects with cocaine disorder. PET (positron emission tomography) was performed using a C-11 radioactive labeled agent binding to κ receptors. Red/yellow indicates higher density of available open κ receptors. Blue/green indicates lower density of available κ receptors.
Baseline on the left demonstrates diffuse distribution and high density of available κ receptors.
Middle represents marked occupation of receptors blocked by the opiate antagonist naltrexone.
On the right following a 3 day cocaine binge there is decrease in open κ receptors compared to baseline. This likely reflects down regulation and resulting cortisol resistance following high volume cocaine use. Strongly negative dysphoric lows in the withdrawal phase are are characteristic of cocaine effects.
Glucocorticoid receptor activity may result in either increased or decreased cortisol and activation of stress pathways. Preclinical animal studies have demonstrated potential for drug therapy targeting glucocorticoid signaling.
Potential targets may involve increasing or inhibiting GC synthesis, receptor agonists or antagonists, or gene expression.
At this time no viable therapeutic agents have emerged targeting these pathways.
……………………………………………………………………
The “dark side” of addiction involves a complex interactive multilevel system of responses to stressful stimuli. The Hypothalamic – Pituitary – Adrenal axis allows the central nervous system to respond to and activate the autonomic system regulating vital functions.
Feedback from corticosteroids, chiefly Cortisol in humans, controls homeostatic balance, controlled response, emotional affective states and has a direct role in consumption of alcohol and other addictive drugs. The extended Amygdala, reward pathway and limbic systems are interconnected functionally related structures.
Attention and therapeutic efforts tend to focus on positive reward and the mesolimbic dopamine reward pathway. While initial use is dominated by positive rewards, as the cycle continues into problematic and compulsive use stress and anxiety mechanisms dominate.
Over expression of cortisol and under expression of regulatory proteins, cortisol resistance, neuropeptide activities contribute to escalating negative affect and allostatic set points. Improved understanding at the molecular level opens up potential for development of new therapeutic approaches.

This post should not be considered medical or professional advice. Images obtained from sources freely available on the World Wide Web. For educational and information purposes only. Comments and suggestions are welcome jeffk072261@gmail.com

References
Dark side
Existing and Future Drugs for the Treatment of the Dark Side of Addiction
George F. Koob and Barbara J. Mason
Committee on the Neurobiology of Addictive Disorders, The Scripps Research Institute,
La Jolla, California 92037; email: mason@scripps.edu, gkoob@scripps.edu
https://future4200.com/uploads/short-url/
Lh4vSwkYpSBY3908P76KTP1wETEj.pdf
Annu. Rev. Pharmacol. Toxicol. 2016. 56:299–322
…………………………………………………………….
The Extended Amygdala and the Dopamine System: Another Piece of the Dopamine Puzzle
11Julie L Fudge, Ana B Emiliano
The Extended Amygdala and the Dopamine System: Another Piece of the Dopamine Puzzle – PMC
……………………………………………………………..
Published in final edited form as: Psychopharmacology (Berl). 2011 Aug 18;218(1):131–156. doi: 10.1007/s00213-011-2443-9
Effects of stress on alcohol drinking: a review of animal studies
Howard C Becker 1,2,3,✉, Marcelo F Lopez 4, Tamara L Doremus-Fitzwater
Effects of stress on alcohol drinking: a review of animal studies – PMC
Effects of Alcohol Dependence and Withdrawal on Stress Responsiveness and Alcohol Consumption
Alcohol Res. 2012;34(4):448–458
Effects of Alcohol Dependence and Withdrawal on Stress Responsiveness and Alcohol Consumption – PMC
…………………………………………..,…..,
, Genome-wide methylation in alcohol use disorder subjects:
implications for an epigenetic regulation of the cortico-limbic
glucocorticoid receptors (NR3C1)
Eleonora Gatta1
Harish R. Krishnan1 Dennis R. Grayson1 Jenny Drnevich
……………………………………………………
NR3C1 Gene – GeneCards | GCR Protein | GCR Antibody
………………………………………………………
Neuropharmacology . 2017 August 01; 122: 136–147. doi:10.1016/j.neuropharm.2017.01.037.
Alcohol, Stress, and Glucocorticoids: From Risk to Dependence
and Relapse in Alcohol Use Disorders
Sara K. Blaine, PhD and Rajita Sinha, PhD
Department of Psychiatry Yale University School of Medicine, Yale
………………………………………………………
Stress and the HPA Axis
Role of Glucocorticoids in Alcohol Dependence
Mary Ann c. Stephens, Ph.D., and Gary Wand, M.D.
Alcohol Research:C u r r e n t R e v i ews
……………………………………………..
A key role for corticotropin-releasing factor in alcohol
dependence
Markus Heilig1 and George F. Koob2,*
1 Laboratory of Clinical and Translational Studies, National Institute of Alcohol Abuse and Alcoholism
(NIAAA), NIH, 10 Center Dr., 1/5334, Bethesda, MD 20892, USA
Trends Neurosci. 2007 August ; 30(8): 399–406. doi:10.1016/j.tins.2007.06.006.
Brain mineralocorticoid and glucocorticoid receptor balance in neuroendocrine regulation and stress-related psychiatric etiopathologies
Edo Ronald de Kloet
Volume 24, June 2022, 100352
……………………………………………………………………..
Adolescent alcohol exposure alters the central brain circuits
known to regulate the stress response
Camryn D Allena,Catherine L Riviera, and Soon Y Leea,
The Salk Institute, The Clayton Foundation Laboratories for Peptide Biology, La Jolla, CA
Neuroscience . 2011 May 19; 182: 162–168. doi:10.1016/j.neuroscience.2011.03.003.
……………………………………………………..
Drug Abuse: Hedonic
Homeostatic Dysregulation
George F. Koob* and Michel Le Moal
SCIENCE VOL. 278 3 OCTOBER 1997 http://www.sciencemag.org
http://www.wisebrain.org/media/Papers/KoobHedonicDysreg_Sci97.pdf
………………………………………………
Evaluation of mifepristone effects on alcohol seeking and self-administration in baboons
August F Holtyn a, Elise M Weerts b
Exp Clin Psychopharmacol. 2018 Dec 20;27(3):227–235. doi: 10.1037/pha000024
Evaluation of mifepristone effects on alcohol seeking and self-administration in baboons – PMC
Corticosteroid sensitization drives opioid addiction
…………………………………………………………..
Alcohol dependence and withdrawal increase sensitivity of central amygdalar GABAergic synapses to the glucocorticoid receptor antagonist mifepristone in male rats
Sophia Khom
Neurobiology of Disease Volume 164 March 2022
……………………………………………………………………….
Hur, J., Kaplan, C.M., Smith, J.F. et al. Acute alcohol administration dampens central extended amygdala reactivity. Sci Rep 8, 16702 (2018). https://doi.org/10.1038/s41598-018-34987-3
Acute alcohol administration dampens central extended amygdala reactivity | Scientific Reports
UStress, alcohol and drug interaction: an update of human research – PM
Stress, alcohol and drug interaction: an update of human research
Magdalena Uhart 1, Gary S Wand 1,2
PMCID: PMC2654253 NIHMSID: NIHMS78923 PMID: 1885580
…………………………………………………
Effects of voluntary ethanol consumption on emotional state and stress responsiveness in socially isolated rats
Maria Giuseppina Pisu c, Maria Cristina Mostallino c, Riccardo Dore a, Elisabetta Maciocco c, Pietro Paolo Secci c, Mariangela Serra a,b,c
Eur Neuropsychopharmacol. 2010 Nov 10;21(5):414–425.j
………………………………………………………………….
Bramham, C.R., Alme, M.N., Bittins, M. et al. The Arc of synaptic memory. Exp Brain Res 200, 125–140 (2010). https://doi.org/10.1007/s00221-009-1959-2
The Arc of synaptic memory | Experimental Brain Research
Corticotropin-releasing factor, neuroplasticity (sensitization), and alcoholism
George F. Koob gkoob@scripps.eduAuthors Info & Affiliations
July 1, 2008
105 (26) 8809-8810
https://doi.org/10.1073/pnas.0804354105
OCorticotropin-releasing factor, neuroplasticity (sensitization), and alcoholism | PNAS
……………………………………………………………..
Stressful life experiences, alcohol consumption, and alcohol use disorders: the epidemiologic evidence for four main types of stressors
Katherine M Keyes 1, Mark L Hatzenbuehler 2, Deborah S Hasin 3
……………………………………………………………..
Molecular and Genetic Substrates Linking Stress and Addiction
Lisa A Briand 1, Julie A Blendy
: Brain Res. 2009 Nov 10;1314C:219.
Molecular and Genetic Substrates Linking Stress and Addiction – PMC
…………………………………………………………….
The Rewarding and Anxiolytic Properties of Ethanol within the Central Nucleus of the Amygdala: Mediated by Genetic Background and Nociceptin
Christopher P Knight 1, Sheketha R Hauser 1, R Aaron Waeiss 1, Andrei I Molosh 1, Philip L Johnson 1,
J Pharmacol Exp Ther. 2020 Sep;374(3):366–375
………………………………………………………………..
Warlow SM, Robinson MJF, Berridge KC. Optogenetic Central Amygdala Stimulation Intensifies and Narrows Motivation for Cocaine. J Neurosci. 2017 Aug 30;37(35):8330-8348. doi: 10.1523/JNEUROSCI.3141-16.2017. Epub 2017 Jul 27. PMID: 28751460; PMCID: PMC5577851.
Optogenetic Central Amygdala Stimulation Intensifies and Narrows Motivation for Cocaine – PMC
………………………………………………………………..
Optogenetics
………………………………………………………………….
Zaveri NT. Nociceptin Opioid Receptor (NOP) as a Therapeutic Target: Progress in Translation from Preclinical Research to Clinical Utility. J Med Chem. 2016 Aug 11;59(15):7011-28. doi: 10.1021/acs.jmedchem.5b01499. Epub 2016 Mar 14. PMID: 26878436; PMCID: PMC5001850.
…………………………………………………………………:
Hur, J., Kaplan bbb bbb Bbb , C.M., Smith, J.F. et al. Acute alcohol administration dampens central extended amygdala reactivity. Sci Rep 8, 16702 (2018). https://doi.org/10.1038/s41598-018-34987-3
Acute alcohol administration dampens central extended amygdala reactivity | Scientific Reports
……………………………………………………………….
Lebow, M., Chen, A. Overshadowed by the amygdala: the bed nucleus of the stria terminalis emerges as key to psychiatric disorders. Mol Psychiatry 21, 450–463 (2016). https://doi.org/10.1038/mp.2016.1
Herman MA, Varodayan FP, Oleata CS, Luu G, Kirson D, Heilig M, Ciccocioppo R, Roberto M. Glutamatergic transmission in the central nucleus of the amygdala is selectively altered in Marchigian Sardinian alcohol-preferring rats: Alcohol and CRF effects. Neuropharmacology. 2016 Mar;102:21-31. doi: 10.1016/j.neuropharm.2015.10.027. Epub 2015 Oct 28. PMID: 26519902; PMCID: PMC4698227.
…………………………………………………………………
Published in final edited form as: Drug Alcohol Depend. 2008 Sep 4;99(1-3):193–203. doi: 10.1016/j.drugalcdep.2008.07.004
Association of Psychiatric and Substance Use Disorder Comorbidity with Cocaine Dependence Severity and Treatment Utilization in Cocaine-Dependent Individuals
Julian D Ford 1, Joel Gelernter 2, Judith S DeVoe 1, Wanli Zhang 1
………………………………………………………………………….
Published in final edited form as: Neuropsychopharmacology. 2006 Jun;31(6):1241–1248. doi: 10.1038/sj.npp.1300872
Social Defeat Stress-Induced Behavioral Responses are Mediated by the Endogenous Kappa Opioid System
Jay P McLaughlin 1, Shuang Li 2, Joseph Valdez 2, Theodore A Chavkin 2, Charles Chavkin 2,*
…………………………………………………………………
…………………………………………………………………….
Amphetamine Regulates Gene Expression in Rat Striatum via
Transcription Factor CREB
Christine Konradi,lJ Rebecca L. Cole, ls3 Stephan Heckers,4 and Steven E. Hymanls*
The Journal of Neuroscience, September 1994, 74(g): 5623-6634
………………………………………………………………………..
Neurobiological mechanisms of addiction: Focus on corticotropin-releasing factor
George F Koob 1,*, Eric P Zorrilla 1
Curr Opin Investig Drugs. 2010 Jan;11(1):63.
Neurobiological mechanisms of addiction: Focus on corticotropin-releasing factor – PMC
………………………………………………………………………
A key role for corticotropin-releasing factor in alcohol dependence
Markus Heilig 1, George F Koob
Trends Neurosci. 2007 Jul 16;30(8):399–406. doi: 10.1016/j.tins.2007.06.006
A key role for corticotropin-releasing factor in alcohol dependence – PMC
…………………………………………………………………………
Corticotropin Releasing Factor–Induced Amygdala Gamma-Aminobutyric Acid Release Plays a Key Role in Alcohol Dependence
Marisa Roberto a Maureen T. Cruz a Nicholas W. Gilpin
Biological Psychiatry May 2010, Pages 831-839
…………………………………………………………………………
Is it really a matter of simple dualism? Corticotropin-releasing factor receptors in body and mental health
Front. Endocrinol., 11 March 2013
Sec. Neuroendocrine Science
Volume 4 – 2013 | https://doi.org/10.3389/fendo.2013.00028
…………………………………………………………………………..,
Conceptual framework for the etiology of alcoholism: a “kindling”/stress hypothesis
George R Breese 1,✉, David H Overstreet 1, Darin J Knapp
Psychopharmacology (Berl). 2004 Oct 23;178(4):367–380. doi:
Conceptual framework for the etiology of alcoholism: a “kindling”/stress hypothesis – PMC
………………………………………………………………………….
Effects of Adrenal Sensitivity, Stress- and Cue-Induced Craving, and Anxiety on Subsequent Alcohol Relapse and Treatment Outcomes
Rajita Sinha 1, Helen C Fox 2, Kwang-ik Adam Hong 3, Julie Hansen 4, Keri Tuit 5, Mary Jeanne Kreek
Arch Gen Psychiatry. 2011 May 2;68(9):942–952. doi: 10.1001/archgenpsychiatry.2011.49
…………………………………………………………………….
Pharmacogenetic approaches to the treatment of alcohol addiction
……………………………………………………………………….
Noradrenergic transmission in the extended amygdala: role in increased drug-seeking and relapse during protracted drug abstinence
Rachel J Smith 1, Gary Aston-Jones
Brain Struct Funct. 2008 Jul 24;213(0):43–61. doi: 10.1007/s00429-
Glucocorticoid Receptor – Endotext – NCBI Bookshelf
…………………………………………………………..
Barr CS, Dvoskin RL, Gupte M, Sommer W, Sun H, Schwandt ML, Lindell SG, Kasckow JW, Suomi SJ, Goldman D, Higley JD, Heilig M. Functional CRH variation increases stress-induced alcohol consumption in primates. Proc Natl Acad Sci U S A. 2009 Aug 25;106(34):14593-8. doi: 10.1073/pnas.0902863106. Epub 2009 Aug 17. PMID: 19706546; PMCID:
https://pmc.ncbi.nlm.nih.gov/articles/PMC2732884/
………………………………………………………………..
Silberman Y, Bajo M, Chappell AM, Christian DT, Cruz M, Diaz MR, Kash T, Lack AK, Messing RO, Siggins GR, Winder D, Roberto M, McCool BA, Weiner JL. Neurobiological mechanisms contributing to alcohol-stress-anxiety interactions. Alcohol. 2009 Nov;43(7):509-19. doi: 10.1016/j.alcohol.2009.01.002. PMID: 19913194; PMCID: PMC2814297.
Neurobiological Mechanisms Contributing to Alcohol-Stress-Anxiety Interactions – PMC
…………………………………………………………………..
Drug-induced stress responses and addiction risk and relapse
Stephanie E. Wemm Rajita Sinha
Neurobiology of Stress February 2019, 100148
Drug-induced stress responses and addiction risk and relapse – ScienceDirect
…………………………………………………………………………..
Sorocco KH, Lovallo WR, Vincent AS, Collins FL. Blunted hypothalamic-pituitary-adrenocortical axis responsivity to stress in persons with a family history of alcoholism. Int J Psychophysiol. 2006 Mar;59(3):210-7. doi: 10.1016/j.ijpsycho.2005.10.009. Epub 2005 Dec 19. PMID: 16360227; PMCID: PMC2267108.
…………………………………………………………………………………
Vanderbilt psychiatry Stress, alcohol, role of central amygdala
……………………………………………………”…………………
Koob – dark side of addiction
Koob neurobiology addiction
………………………………………………………………….
Göver, T., Slezak, M. Targeting glucocorticoid receptor signaling pathway for treatment of stress-related brain disorders. Pharmacol. Rep 76, 1333–1345 (2024). https://doi.org/10.1007/s43440-024-00654-w
Traumatic Stress-Induced
Vulnerability to Addiction: Critical Role
of the Dynorphin/Kappa Opioid
Receptor System
Frontiers in Pharmacology | http://www.frontiersin.org 1
April 2022 | Volume 13 | Article 85667
Claire Leconte, Raymond Mongeau and Florence Noble *
Université Paris Cité, INSERM, CNRS, T3S, Paris, France
https://www.frontiersin.org/journals/pharmacology/articles/10.3389/fphar.2022.856672/pdf
……………………………………………………………………….
Dynorphin/kappa opioid receptor system regulation on amygdaloid circuitry: Implications for neuropsychiatric disorders
………………………………………………………………………
Pharmacological Evidence for a Motivational Role of k-Opioid
Systems in Ethanol Dependence
Brendan M Walker*,1 and George F Koob1
1Committee on the Neurobiology of Addictive Disorders, The Scripps Research Institute, La Jolla, CA, USA
Neuropsychopharmacology (2008) 33, 643–652
Kappa-opioid receptors, dynorphin, and cocaine addiction: a positron emission tomography study
Neuropsychopharmacology. 2019 Apr 26;44(10):1720–1727. doi: 10.1038/s41386-019-0398-4
Kappa-opioid receptors, dynorphin, and cocaine addiction: a positron emission tomography study – PMC
……………………………………………………………………..
………………………………………………………………………
JK 3/25


Leave a comment